Figures & data
Table 1 Pharmacological characteristics and use in the clinical setting in Japan and efficacy of DPP-4 inhibitors in patients with type 2 diabetes based on the data for Phase III clinical trials
Figure 1 Prescription rates of antidiabetic agents.
Abbreviations: DPP-4, dipeptidyl peptidase-4; GLP-1, glucagon-like peptide-1.
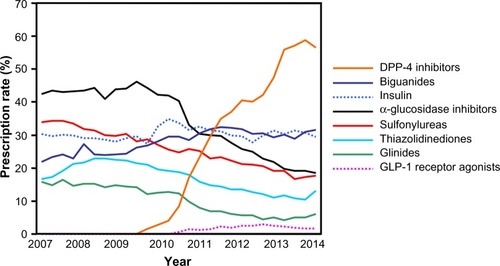
Figure 2 Data for (A) patient age, (B) estimated glomerular filtration rate (eGFR), (C) urinary C-peptide, and (D) therapeutic methods for type 2 diabetes among the groups divided according to the duration of illness.
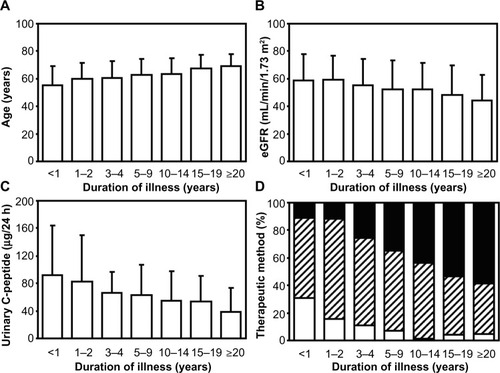
Table 2 Changes in the levels of HbA1c, fasting blood glucose, and postprandial blood glucose in the groups treated with combination therapy with anagliptin and other oral antidiabetic agents in a Phase III clinical trial
Table 3 Changes in the serum lipid concentrations in a Phase III trial
Table 4 Side effects observed in more than 2% of subjects treated with anagliptin and other oral antidiabetic agents in a Phase III trial in Japan
