Figures & data
Figure 1 Selection of trials for pooled analysis and patient disposition. Of the 16 completed clinical trials with available data, eight comparator-controlled studies were included.
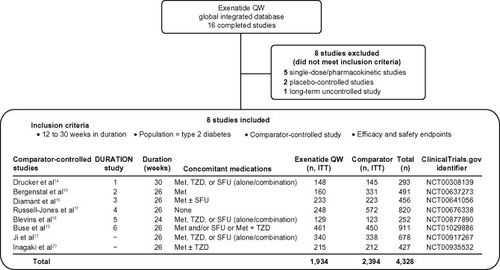
Table 1 Baseline demographics
Table 2 Summary of serious AEs, discontinuations, and deaths for DURATION-1–6 and Asian studies
Table 3 Summary of serious AEs, discontinuations, and deaths for the DURATION-6 study
Table 4 Summary of frequent (≥5%) treatment-emergent adverse events for DURATION-1–6 and Asian studies
Table 5 Summary of frequent (≥5%) treatment-emergent adverse events for the DURATION-6 study
Figure 2 Incidence and duration of gastrointestinal-related adverse events over time. Incidence (left panel) and duration (right panel) for (A, B) nausea, (C, D) vomiting, and (E, F) diarrhea in each treatment group. Duration of the nausea/vomiting event is calculated as the resolution date (or the last participation date if event is ongoing at the time of study termination) minus the event onset date plus 1. aEvents lasting longer than 7 days in duration included reports of both continuous and intermittent nausea/vomiting. 95% confidence interval for the difference (exenatide QW incidence [%] minus liraglutide QD incidence [%] in DURATION-6).
![Figure 2 Incidence and duration of gastrointestinal-related adverse events over time. Incidence (left panel) and duration (right panel) for (A, B) nausea, (C, D) vomiting, and (E, F) diarrhea in each treatment group. Duration of the nausea/vomiting event is calculated as the resolution date (or the last participation date if event is ongoing at the time of study termination) minus the event onset date plus 1. aEvents lasting longer than 7 days in duration included reports of both continuous and intermittent nausea/vomiting. 95% confidence interval for the difference (exenatide QW incidence [%] minus liraglutide QD incidence [%] in DURATION-6).](/cms/asset/a7fb2760-3a88-4815-93f9-d9d0cbcbd3b7/dmso_a_77290_f0002_c.jpg)
Figure 3 Occurrence of gastrointestinal-related adverse events over time with exenatide QW treatment. Incidences of nausea, vomiting, and diarrhea in patients treated with exenatide QW are combined for weeks 0–12 in 2-week increments.Citation14–Citation21
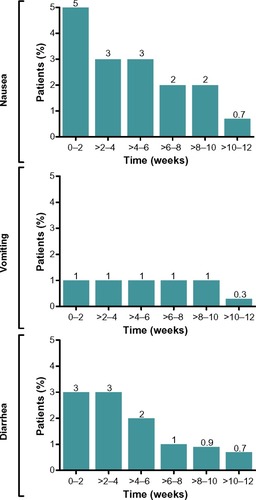
Figure 4 Incidence of hypoglycemia by treatment and use of SFU. Percentage of patients who experienced minor hypoglycemia. 95% confidence interval of the difference (exenatide QW incidence [%] minus liraglutide QD incidence [%] in DURATION-6).
![Figure 4 Incidence of hypoglycemia by treatment and use of SFU. Percentage of patients who experienced minor hypoglycemia. 95% confidence interval of the difference (exenatide QW incidence [%] minus liraglutide QD incidence [%] in DURATION-6).](/cms/asset/3552d917-acc6-4d7c-81b8-47221b8e89c7/dmso_a_77290_f0004_c.jpg)
Figure 5 Adverse events of interest. Exposure-adjusted incidence per 100 patient-years and difference in thyroid neoplasm, pancreatitis, renal failure, and gallbladder disease. Pancreatitis includes acute pancreatitis and chronic pancreatitis. Thyroid neoplasm includes benign neoplasm of the thyroid gland and malignant thyroid neoplasm. 95% confidence interval for the difference (exenatide QW incidence [%] minus liraglutide QD incidence [%] in DURATION-6).
![Figure 5 Adverse events of interest. Exposure-adjusted incidence per 100 patient-years and difference in thyroid neoplasm, pancreatitis, renal failure, and gallbladder disease. Pancreatitis includes acute pancreatitis and chronic pancreatitis. Thyroid neoplasm includes benign neoplasm of the thyroid gland and malignant thyroid neoplasm. 95% confidence interval for the difference (exenatide QW incidence [%] minus liraglutide QD incidence [%] in DURATION-6).](/cms/asset/29d92d78-5950-424e-a7c9-a14c1b586c13/dmso_a_77290_f0005_c.jpg)
Table 6 Treatment-emergent serious adverse events by body system (>1% in any group) for 24-week to 30-week randomized comparator-controlled trialsCitation14–Citation21
