Figures & data
Table 1 Baseline characteristics of study participants that completed the 24-week study
Figure 1 Disposition of participants in this study.
Abbreviations: ITT, intent-to-treat; PI, principal investigator.
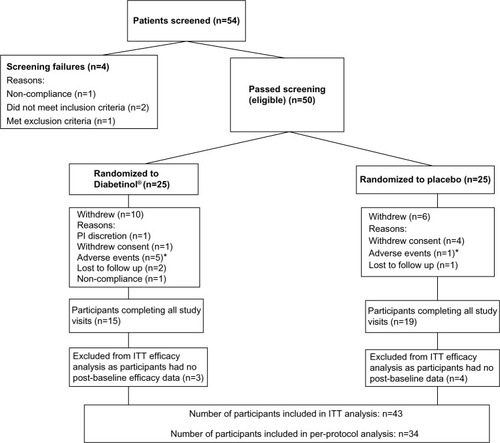
Table 2 Glycemic parameters of study participants that completed the 24-week study
Figure 2 Four-hour postprandial serum glucose and insulin levels after supplementation with placebo or Diabetinol®.
Abbreviations: min, minutes; SD, standard deviation.
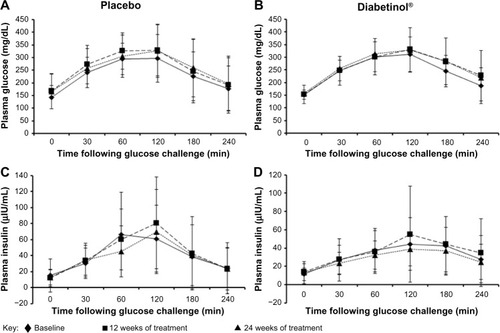
Figure 3 Change from baseline in glycemic parameters after supplementation with placebo or Diabetinol®.
Abbreviations: FBG, fasting blood glucose; HbA1c, hemoglobin A1c; PP, postprandial; SD, standard deviation.
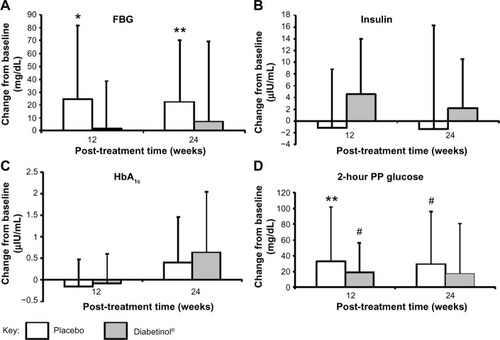
Figure 4 Four-hour postprandial serum glucose levels after supplementation with placebo or Diabetinol®.
Abbreviations: min. minutes, SD, standard deviation.
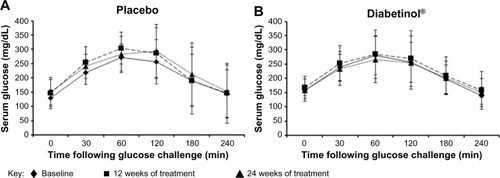
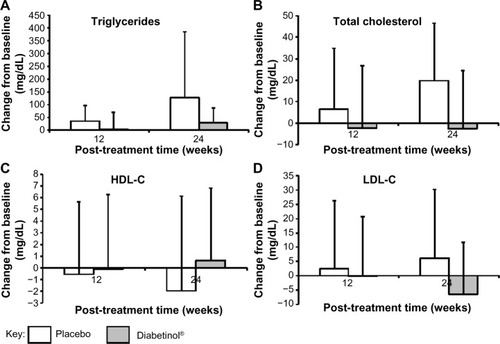
Figure 5 Change from baseline in blood lipid parameters after supplementation with placebo or Diabetinol®.
Abbreviations: HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SD, standard deviation.