Figures & data
Table 1 Options and considerations for patient population selection in an outcome study
Figure 1 Issues in research program design.
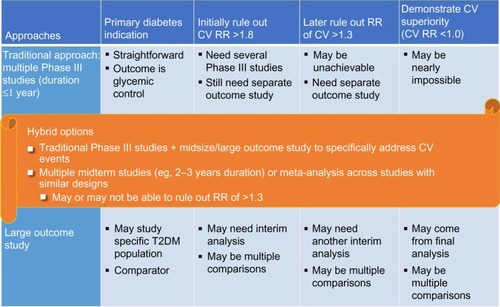
Table 2 Selected type 2 diabetes mellitus CV outcome studies
Figure 2 Graphic illustration of different trial plans and data analysis strategies.
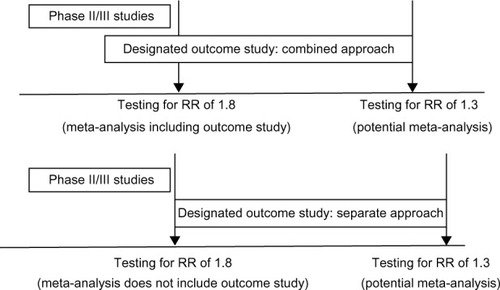
Table 3 Study design and testing strategy for selected CV outcome studies
Table S1 Patient population/risk factor comparison for outcome studies
Table S2 Endpoint selection for CV safety evaluation
