Figures & data
Table 1 Mean (±standard deviation) single-dose pharmacokinetic parameters of immediate-release formulations of 4-aminopyridine (4-AP) and dalfampridine extended release 10 mg tablets
Figure 1 Proportion of timed 25-foot walk (T25FW) responders in the pooled analysis and component studies (modified intent-to-treat population).Citation67
Abbreviation: MS, multiple sclerosis; ER, extended release.
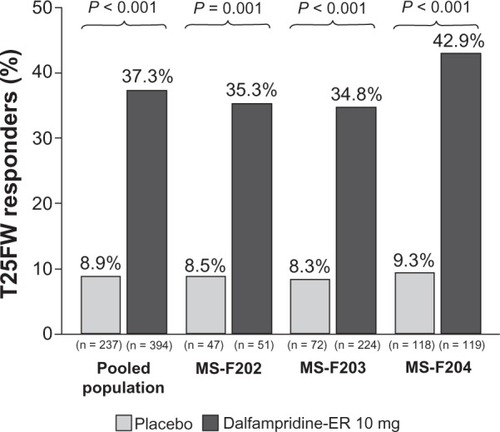
Figure 2 Average percent change from baseline during the treatment period in walking speed among dalfampridine extended release (ER) responders relative to placebo group and dalfampridine-ER nonresponders in the pooled analysis and component studies (modified intent-to-treat population).Citation66
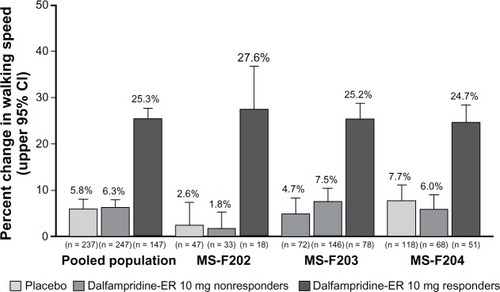
Figure 3 Pooled analysis of timed-walk responder rate stratified by baseline demographic characteristics (modified intent-to-treat population).Citation67 (A) Gender. (B) Race. (C) Age. (D) Body mass index.
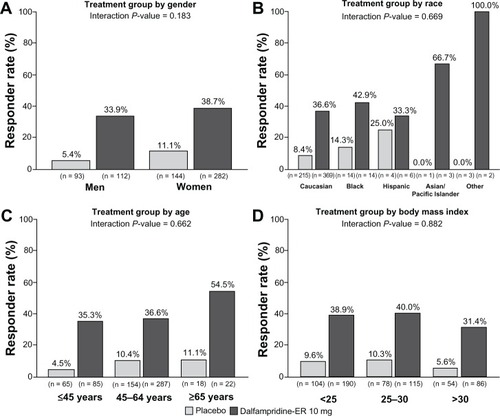
Figure 4 Pooled analysis of timed-walk responder rate stratified by multiple sclerosis (MS) disease characteristics (modified intent-to-treat population).Citation67 (A) MS type. Relapsing remitting (RR), secondary progressive (SP), primary progressive (PP), progressive relapsing (PR). Because of the low number of patients with progressive-relapsing MS, the analysis of treatment by MS disease course type was computed post hoc, using only the RR, PP, and SP patients. (B) MS duration stratified by quartiles: Q1, ≥0.1 to <6.2 years; Q2, ≥6.2 to <10.8 years; Q3, ≥10.8 to <15.5 years; Q4, ≥15.5 years to 45.6 years (maximum).
Figure 5 Percent change from baseline in walking speed in the parent studies and open-label long-term extensions by double-blind dalfampridine extended release (ER) responder status. (A) MS-F203. (B) MS-F204.
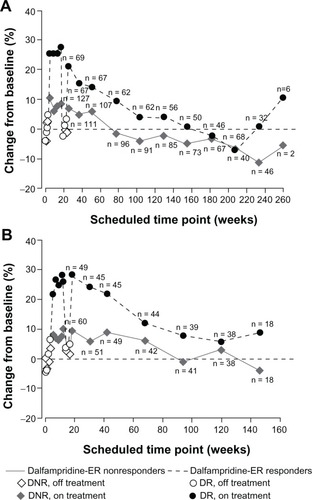
Figure 6 Percent change from baseline in walking speed by double-blind dalfampridine-ER responder status among those patients with continuous participation. (A) At week 104 in MS-F203. (B) At week 96 in MS-F204.
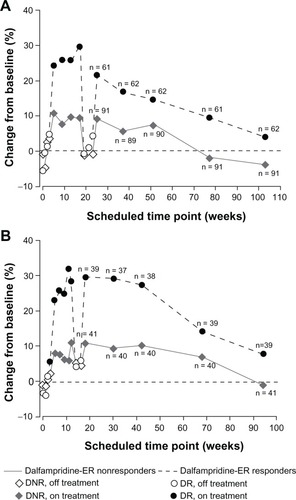
Table 2 Treatment-emergent adverse events (TEAEs) that occurred in ≥5% of dalfampridine extended release (ER)-treated patients in the pooled MS-F202, MS-F203, and MS-F204 safety populationTable Footnotea