Figures & data
Table 1 Treatment-Emergent Adverse Events (>5% and of Special Interest) in the Pooled Analysis of Four Clinical Trials of Istradefylline Versus Placebo
Figure 1 Reduction in OFF time and increases in good ON time (ON without troublesome dyskinesia) from the four randomized controlled studies (istradefylline versus placebo) that led to FDA approval. Study 1, 6002-US-005;Citation25 Study 2, 6002-US-013; Study 3, 6002-0608;Citation30 Study 4, 6002-009.Citation31
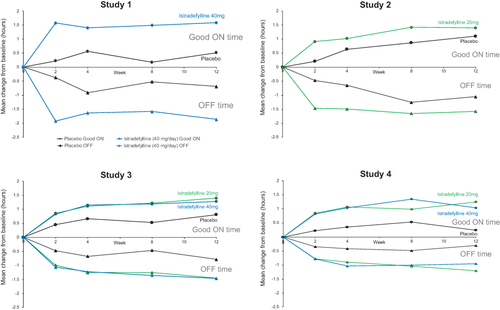
Figure 2 Summary of efficacy outcomes from the four randomized controlled studies (istradefylline versus placebo) that led to FDA approval. Study 1, 6002-US-005;Citation25 Study 2, 6002-US-013;40 Study 3, 6002-0608;Citation30 Study 4, 6002-009.Citation31
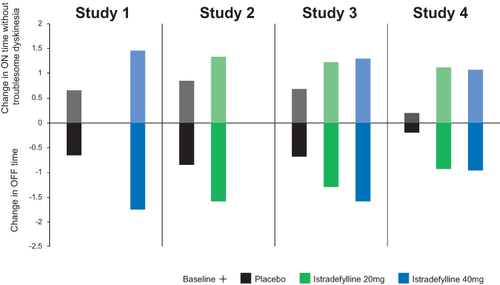
Table 2 Post-Marketing Clinical Research to Evaluate Parkinson’s Disease Symptoms in Japan
