Figures & data
Figure 1 Impaired insulin signaling/ secretion involved in the progression of dementia This schematic diagram depicts both normal and abnormal insulin signaling in the brain. Insulin activates signaling pathways such as AKT, PI3K, JNK, NF-KB, GLUT4 and GSK3. Synaptic plasticity and memory are both linked to the PI3K pathway. The PI3K pathway is linked to synaptic plasticity and memory. As a result, aberrant insulin binding can cause neuroinflammation by activating proinflammatory cytokines and lowering cognitive capacities via JNK and NF-KB. Long-term memory loss is caused by oxidative stress, mitochondrial dysfunction, and inflammation caused by GSK3 and its hyperphosphorylation. It also elevates the expression of caspase-3 and 9, which ultimately leads to neuronal cell death.
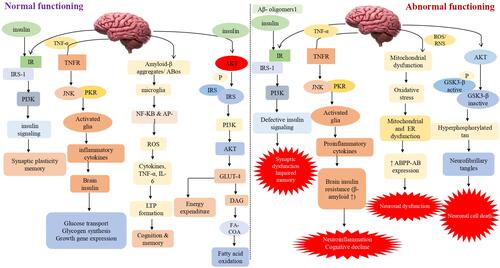
Figure 2 Insulin resistance in dementia is represented schematically by mitochondrial dysfunction, which leads to synaptic damage and neurodegeneration, glycosylated haemoglobin in intoxicated cognitive function due to failure in glucose transport for neurons, oxidative stress-induced amyloid beta and phosphorylated tau lineups via advanced glycation end products, inflammation caused by mitochondrial dysfunction and toxicities of amyloid-beta and glycation end products. Additionally, in both normal and impaired insulin signaling, Tau hyperphosphorylation was also observed, stimulates AKT, and inhibits tau hyperphosphorylation by inactivating GSK-3. Furthermore, the TOR pathway was also active for cellular growth and kept autophagy at a low level for cellular function. Black normal flow lines represent normal working mechanism; dotted red lines show inhibition of the pathway, and normal dark red flow lines show the overall cause of dementia.
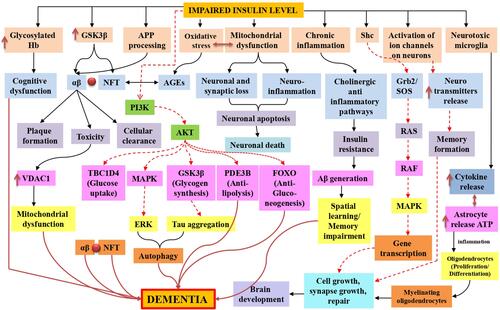
Table 1 Impaired Insulin Signaling Dysfunction in the Progression of Dementia and Related Neurological Complications
Table 2 Neuroprotective Role of GLP-1 Signaling Analogs in the Protection of Various Dementia Related Neurodegenerative Abnormalities
