Figures & data
Table 1 Consistency of clinical and radiological outcomes between oral therapies compared to current licensed therapies for RRMS; not from comparative studies
Figure 1 Neuroprotective mode of action: MMF on Nrf2 Phase II genes activation, such as NQO1 (NAD(P)H:quinone oxidoreductase-1) and glutathione S-transferase.
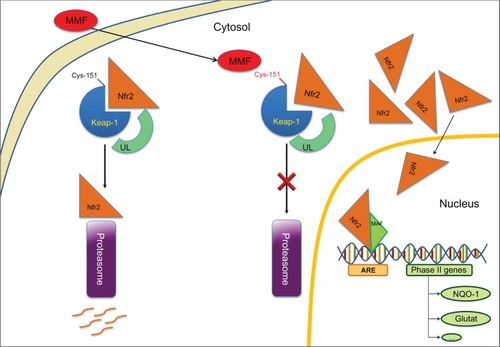
Figure 2 Anti-inflammatory mode of action: DMF on NF-kB activation.
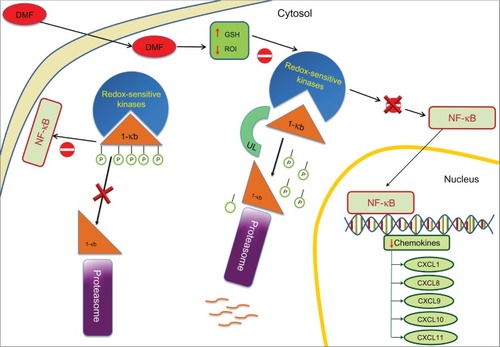
Table 2 Efficacy results of BG-12 in DEFINECitation84,Citation85 and CONFIRMCitation86 Phase III studies
Figure 3 Reduction of ARR at 2 years by BG-12 and DMTs approved for MS treatment, as reported in Phase III clinical trials.
Abbreviations: ARR, annualized relapse rate; DMT, disease-modifying treatment; MS, multiple sclerosis; BID, twice a day; TID, three times a day.
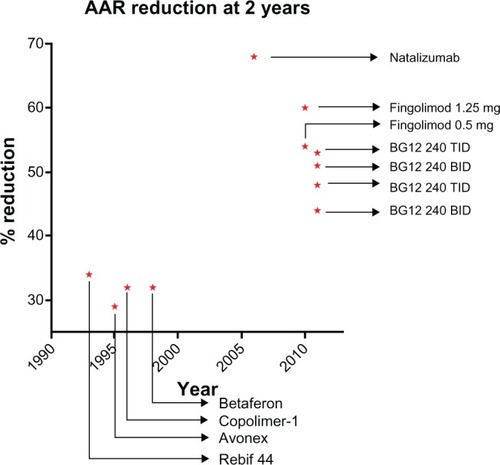
Table 3 Adverse events reported in Phase IIbCitation82 and DEFINECitation88 trials
Table 4 Relative efficacy (reduction in ARR), safety (major side effects) and ease of use (administration, day-to-day side effects) of existing and emerging therapies currently applying for a USA/EU license; not from comparative studies