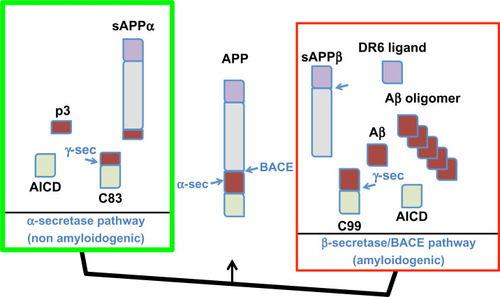
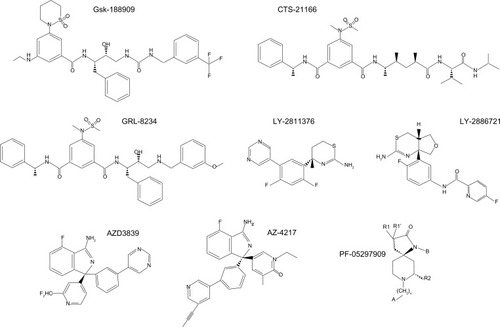
Figures & data
Table 1 Data of BACE1 inhibitors in clinical trials
Table 2 List of identified BACEI substrates
Table 3 Identified BACE1 substrate-cleavage sites