Figures & data
Figure 1 (A–J) Relationships among single-unit tuning, local field potential (LFP) voltage, and LFP power.
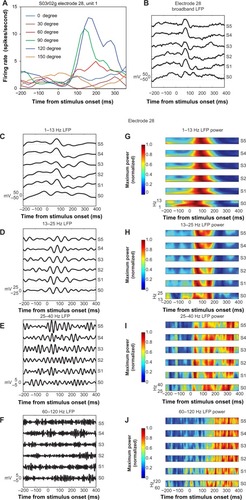
Figure 2 (A–C) Microelectrode-array electrode positions relative to cytochrome oxidase stripes.
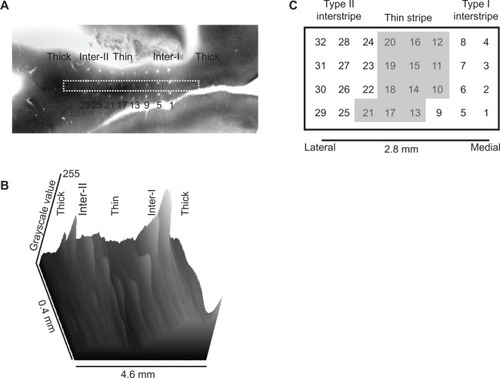
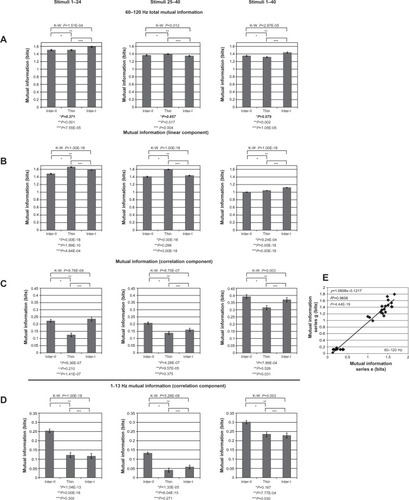
Figure 3 Linear component of mutual information encoded by local field potential (LFP) power in four frequency bands.
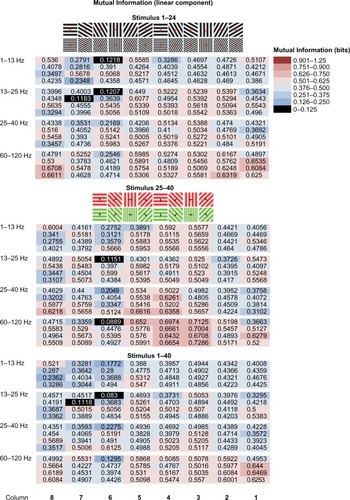
Figure 4 (A–C) Stimulus-dependent distribution of linear MI across the array.
Abbreviations: MI, mutual information; LFP, local field potential; sf, spatial frequency; diff, difference.
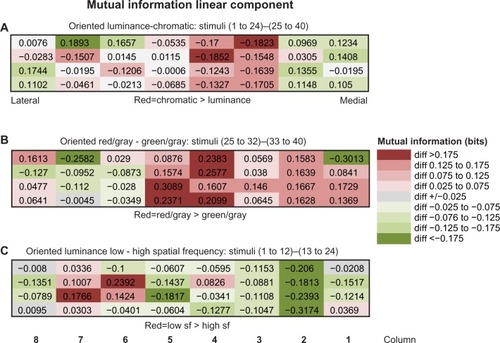
Figure 5 (A and B) Noise correlations as a function of single-unit cortical separation.
Notes: (A) The relationship between noise correlation (correlated variability) and single-unit-pair cortical separation was found to depend on the orientation preferences of the unit pairs. Unit pairs with preferred orientations differing by less than 60° showed the expected decrease in noise correlations with increased distance (blue). Nearby neurons showed high noise correlations (~0.2), while pairs separated by 2 mm had low correlations (~0.05). A similar pattern was observed for neuron pairs spanning the two interstripes with orientation differences >60° (red). Surprisingly, other neuron pairs with orientation differences >60° showed an increase in noise correlations with distance. (B) Relationship between unit noise correlations and cortical separation during stimulation with isoluminant hue patches. Noise correlations were greatest for units recorded at the same electrode (~0.3) and decreased rapidly with cortical separation.
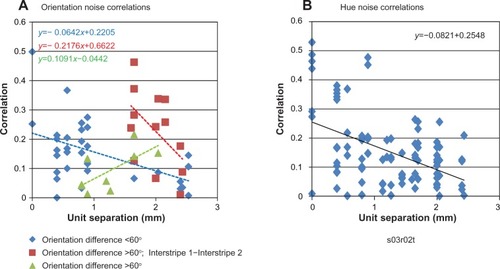
Figure 6 (A and B) Mutual information (MI) varies with reference-electrode position.
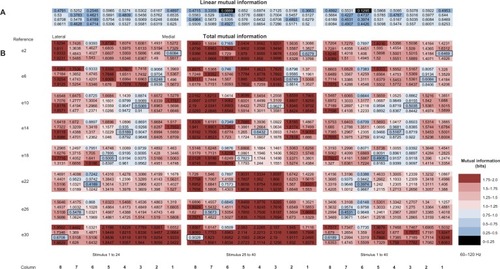
Figure 7 (A–D) Distribution of mutual information (MI) components due to interactions with the reference electrode.
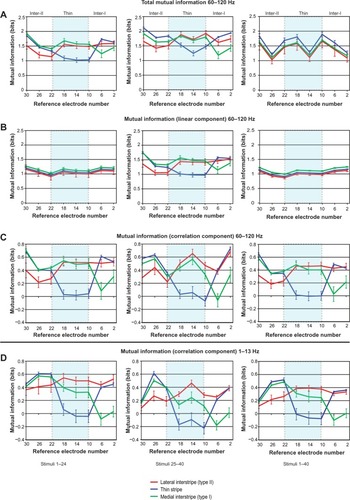
Figure 8 (A–D) Mutual information (MI) in cytochrome oxidase (CO) stripes varied systematically due to interstripe interactions.
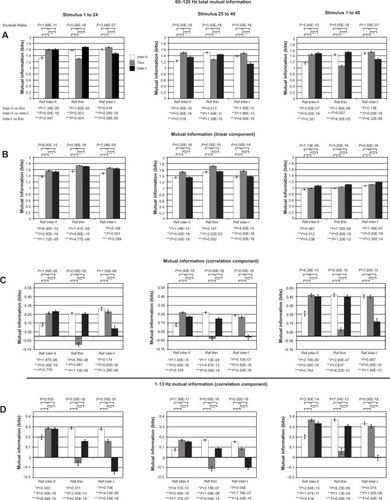
Figure 9 (A–E) Mutual information (MI) due to interstripe interactions.