Figures & data
Figure 1 Vasculogenesis in the newborn dog.
Abbreviation: ADPase, adenosine diphosphatase.
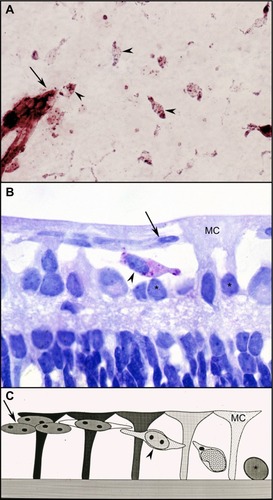
Figure 2 Vaso-obliteration in the dog model of OIR.
Abbreviations: OIR, oxygen-induced retinopathy; ADPase, adenosine diphosphatase.
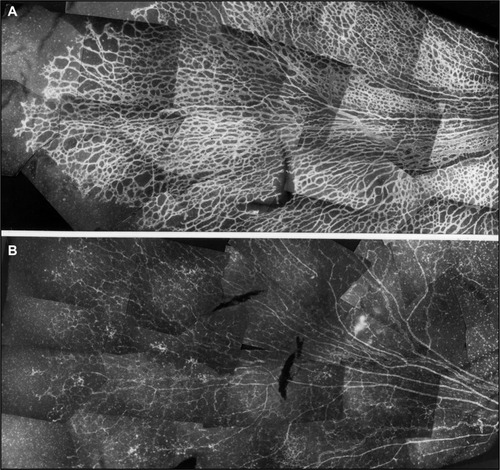
Figure 3 Hyperoxia affects all regions uniformly in the dog OIR model.
Abbreviation: OIR, oxygen-induced retinopathy.
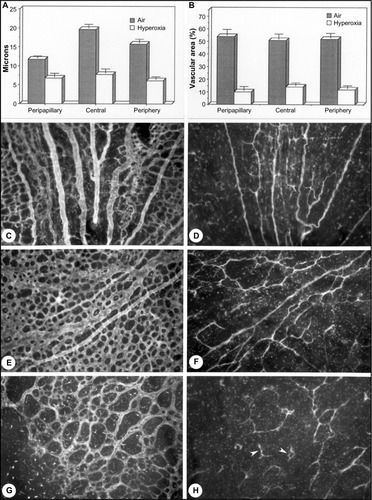
Figure 4 Vasoproliferative phase of OIR in dog.
Abbreviation: OIR, oxygen-induced retinopathy.
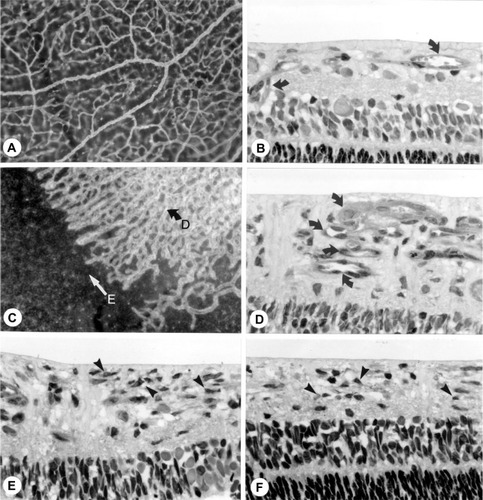
Figure 5 Intravitreal neovascularization in the dog OIR model.
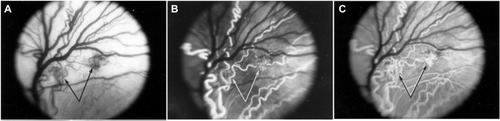
Figure 6 Possible sequence leading to the evolution of inter-anastomosing neovascular networks in the vitreous from a 22-day-old oxygen-treated animal.
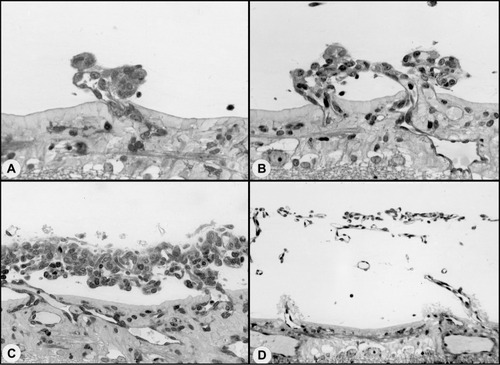
Figure 7 Clinical appearance of vascularized membranes in oxygen-treated animals.
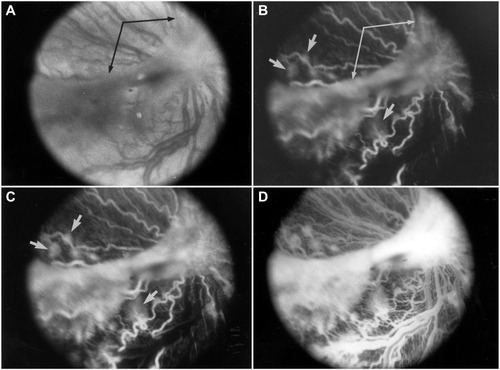
Figure 8 Tented vascularized membrane and tractional retinal folds in a 45-day-old oxygen-treated animal.
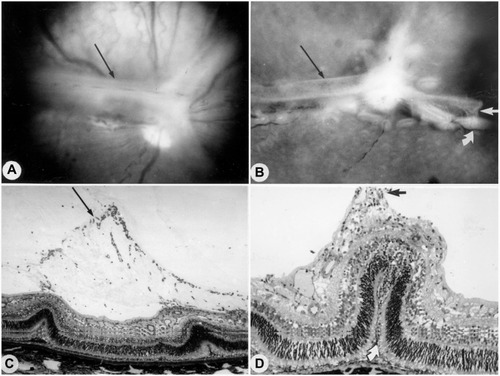
Figure 9 KDR (VEGFR2) localizationin the canine OIR model.
Abbreviations: vWf, von Willebrand’s factor; KDR, kinase domain receptor; NV, neovascularization.
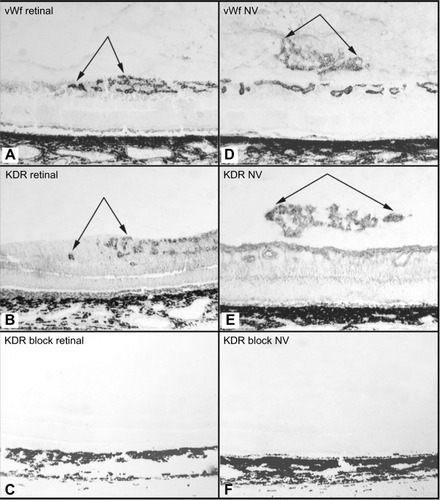
Figure 10 Effect of anti-KDR on dog OIR.
Abbreviations: OIR, oxygen-induced retinopathy; ADPase, adenosine diphosphatase; KDR, kinase domain receptor.
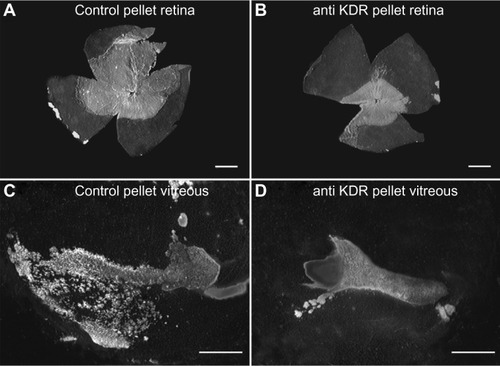
Figure 11 Effect of anti-KDR on intravitreal and retinal vascular area.
Abbreviation: KDR, kinase domain receptor.
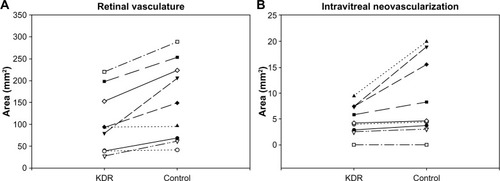
Figure 12 Retinal vasculature and intravitreal neovascularization after treatment with VEGF-Trap.
Abbreviation: ADPase, adenosine diphosphatase.
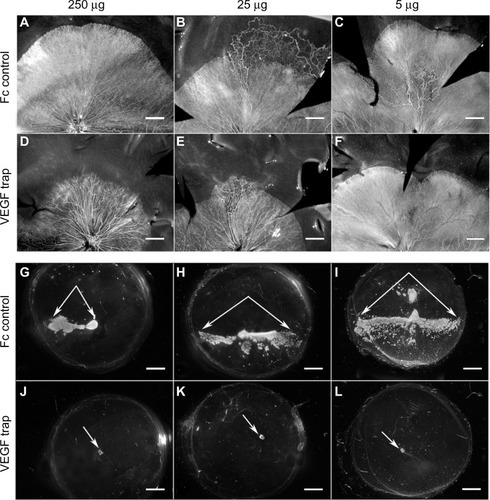
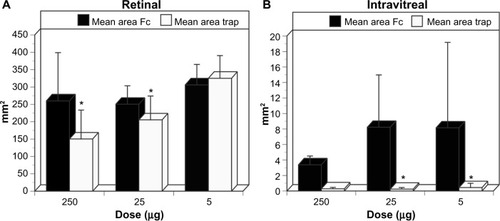
Figure 13 Retinal and intravitreal vasculature areas after treatment with VEGF-Trap
Abbreviation: NV, neovascularization.