Figures & data
Figure 1 Map of regional changes in human foveal development by age from 31 weeks PMA until adulthood.
Abbreviations: IRL, inner retinal layer; OS, outer segment; PMA, postmenstrual age; PRL, photoreceptor layer; ROP, retinopathy of prematurity; SDOCT, spectral domain-optical coherence tomography; TRL, total retinal layer.
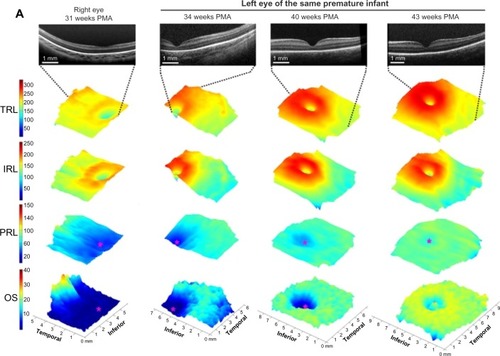
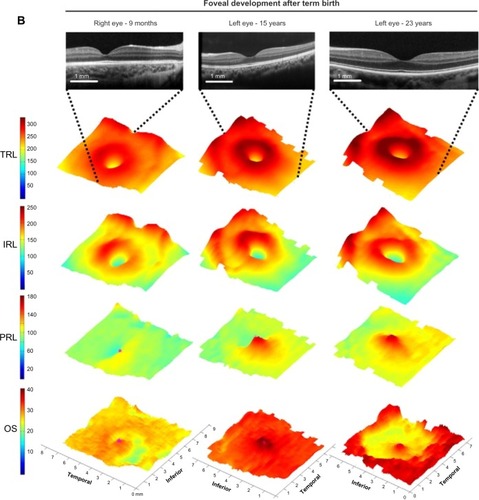
Figure 2 SDOCT histology comparison of normal premature infant retinas.
Abbreviations: ELM, external limiting membrane; GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; IS, inner segment; ISE, inner segments ellipsoid; NFL, nerve fiber layer; ONL, outer nuclear layer; OPL, outer plexiform layer; OS, outer segment; PMA, postmenstrual age; RPE, retinal pigment epithelium; SDOCT, spectral domain optical coherence tomography.
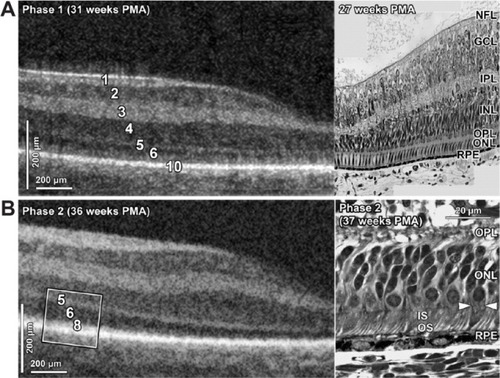
Figure 3 Morphologic characteristics and phenotypes of CME observed by use of SDOCT.
Abbreviations: CME, cystoid macular edema; SDOCT, spectral domain optical coherence tomography.
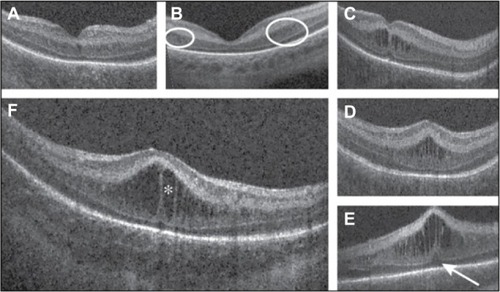
Figure 4 Evaluation of infant retinal vasculature with SDOCT.
Abbreviations: PMA, postmenstrual age; SDOCT, spectral domain optical coherence tomography.
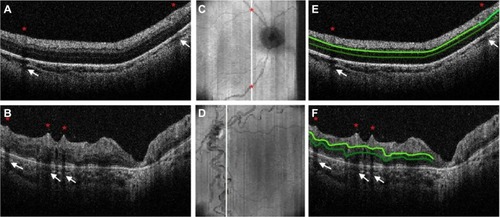
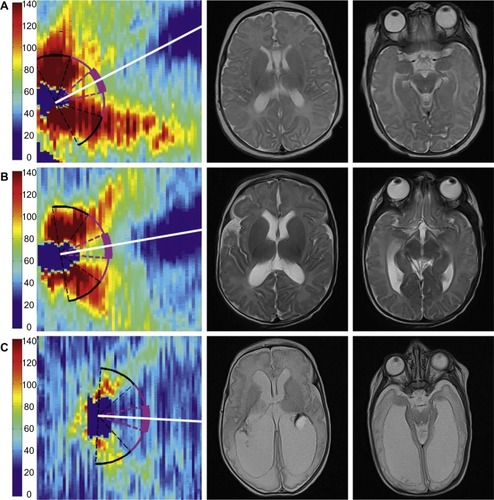
Figure 5 Very preterm infant RNFL thickness maps at term equivalent age (left column) and their corresponding near-term T2-weighted brain MRI (B, right columns).
Abbreviations: MRI, magnetic resonance imaging; RNFL, retinal nerve fiber layer.