Figures & data
Table 1 Reports of refractive error after the use of anti-vascular endothelial growth factor agents for the treatment of retinopathy of prematurity
Figure 1 Zone I distribution of refractive error by treatment modality.
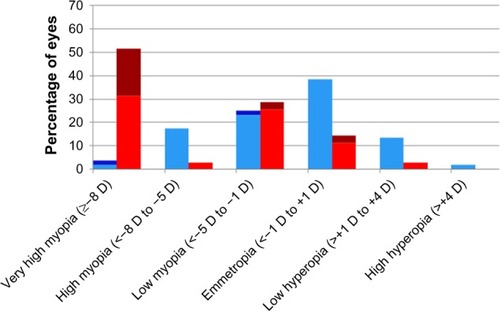
Figure 2 Zone II posterior distribution of refractive error by treatment modality.
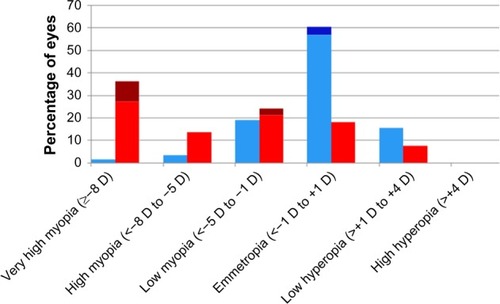
Table 2 Cycloplegic retinoscopic refractive error at age 2.5 years in the Bevacizumab Eliminates the Angiogenic Threat for Retinopathy of Prematurity clinical trialCitation1
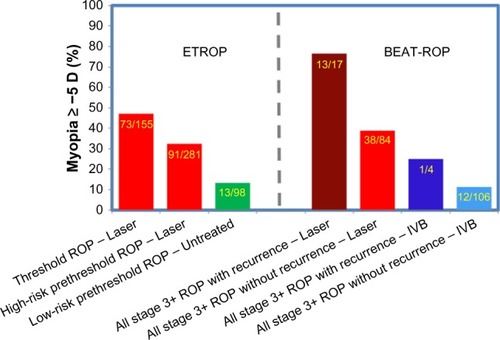
Figure 3 Percentage of eyes ≥−5 D in ETROP and BEAT-ROP by ROP severity at treatment.
Abbreviations: ROP, retinopathy of prematurity; IVB, intravitreal bevacizumab.