Figures & data
Table 1 Characteristics of the 21 patients with HER2-positive AGC receiving (11) versus not receiving (10) TBP
Figure 1 Median OS (A) and PFS (B) of the second-line TBP therapy.
Abbreviations: OS, overall survival; PFS, progression-free survival; TBP, trastuzumab beyond progression.
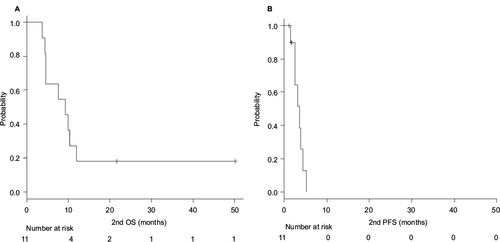
Figure 2 Median OS (A and B) and PFS (C) after starting the first-line therapy.
Abbreviations: OS, overall survival; PFS, progression-free survival; TBP, trastuzumab beyond progression.
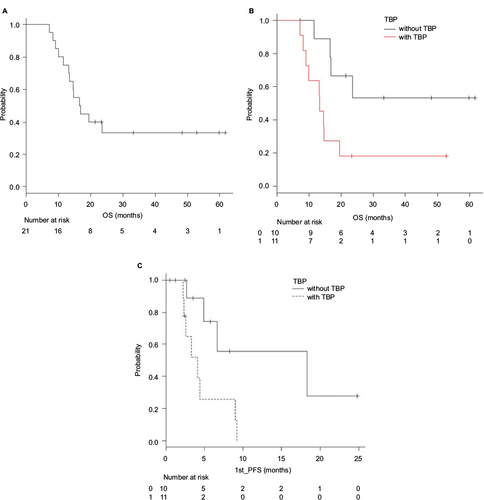
Figure 3 The PFS after the first-line chemotherapy, the duration of TBP and the OS are depicted as bar charts, in addition to the clinical outcomes of the first-line chemotherapy.
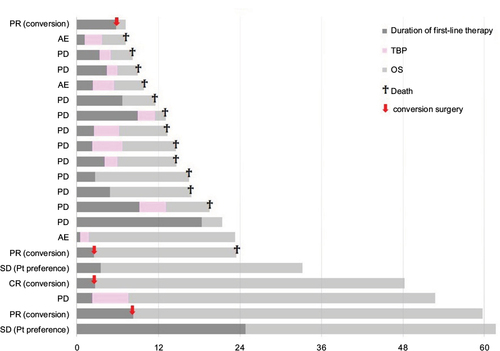
Table 2 Hematologic adverse events of patients receiving TBP
Table 3 Outcomes of clinical trials for patients with HER2-positive AGC
