Figures & data
Figure 1 Natural progression of liver disease. Liver insult of any etiology results in inflammatory changes in hepatic parenchyma which progress to fibrosis and ultimately cirrhosis if unaddressed. Cirrhosis culminates in liver failure and is a principal risk factor for hepatocellular carcinoma.
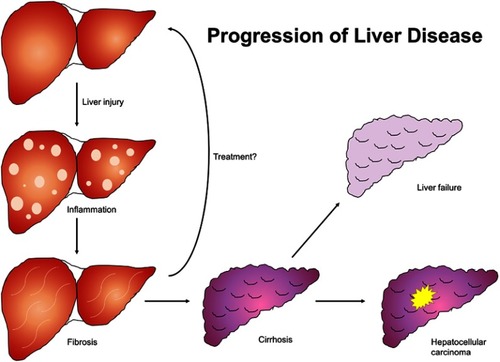
Figure 2 Extracellular signaling mechanisms initiated by and contributing to hepatic stellate cell activation. Activation of HSCs and initiation of fibrogenic, contractile, proliferative and chemotactile processes facilitate perpetuation of fibrogenesis. Chemotaxis through synthesis and secretion of various growth factors, such as CTGF (SWISS-MODEL accession P29279), VEGF (PDB ID 1TZH),Citation226 PDGF (PDB ID 4QCI)Citation227 and EGF (PDB ID 2KV4),Citation228 promote proliferation, secretion of ECM components and maintenance of a profibrotic milieu via juxtacrine, paracrine and autocrine interactions. Upregulation of fibrogenic genes, including αSMA (red chains) to enable contractility and collagen III (PDB ID 6A0A),Citation229 leads to expansion of ECM and fibrotic septa, and integrin-dependent matricrine interactions facilitate perpetuation of the activated state. Dysregulation of matrix degradation through differential expression of various MMPs, including MMP9 (PDB ID 1L6J),Citation230 enables accumulation of ECM components and sustenance of a profibrotic microenvironment. Inflammatory signaling recruits various WBCs and macrophages with profibrotic downstream effectors through Th1 and Th17 juxtacrine and paracrine signaling. Activated HSCs proliferate via TGF-β-, Ras- and Hedgehog-dependent pathways. Resolution of HSC activation proceeds via apoptosis, senescence or reversal to a quiescent state; it is unclear whether reversal to the deactivated state fully occurs. Crystal structures rendered with NGL Viewer.Citation231
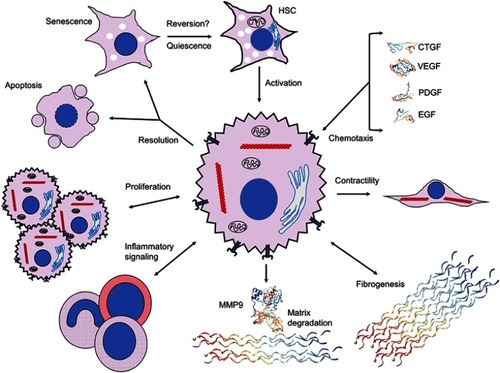
Figure 3 Intracellular signaling pathways involved in initiation and perpetuation of hepatic stellate cell activation. Initiation of the activated state involves engagement of proliferative and activating pathways, including TGFβR (PDB IDs 1KTZ, 3TZM, 5E8U, 5E8V)Citation232–Citation234 binding and downstream nuclear translocation of Smad2/3 and Smad 4 proteins, resulting in transcription and translation of profibrotic genes. Binding of PDGF to its receptor PDGFR (PDB ID 5GRN)Citation235 results in activation of cytoplasmic Ras and MAPK effectors, with subsequent nuclear translocation and expression of proliferative genes. Engagement of the GPCR CCR5 (PDB ID 6AKX)Citation236 results in clathrin-mediated endocytosis, activation and nuclear translocation of ERK1/2, and expression of proliferative genes, also allowing for migratory properties. Cytokines also have significant modulatory properties on fibrogenesis; for example, binding of IL-17 to its receptor IL-17R (PDB ID 4HSA)Citation237 results in activation of the canonical NF-κB pathway, resulting in pro-inflammatory gene expression. Programmed cell death can be facilitated via innate immune signaling; engagement of TLRs (PDB IDs 6NIH, 2MKA, 2J67)Citation238–Citation240 with various TLR ligands propagates downstream signaling culminating in autophagy, apoptosis or pyroptosis. Nuclear membrane receptors also have large roles in modulating profibrogenic properties; engagement of ligands, including fatty acids and thiazolidinediones, with receptors of the PPAR family, including PPARγ (PDB ID 2Q8S),Citation241 results in nuclear translocation, heterodimerization with RXR and binding with DNA (PDB ID 3DZU),Citation242 with subsequent expression of antifibrogenic genes. Binding of obeticholic acid to FXR and of vitamin D to VDR results in receptor nuclear translocation, heterodimerization with RXR and expression of antifibrogenic genes. Meanwhile, binding of heme to Rev-Erb upregulates Rev-Erb expression, accumulation of Rev-Erb in the cytoplasm and promotion of a profibrogenic, contractile HSC phenotype. Crystal structures rendered with NGL Viewer.Citation231
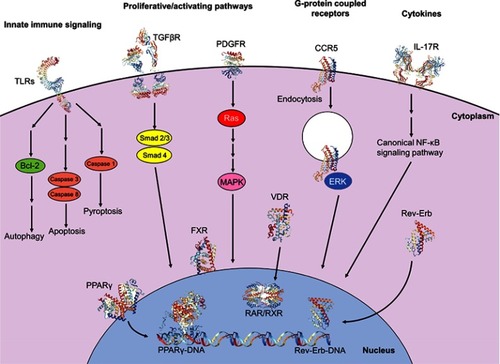
Table 1 Summary of published and ongoing phase II/III randomized controlled trials (RCTs) of therapeutic agents for hepatic fibrosis reversal
