Figures & data
Figure 1 Patient Disposition.
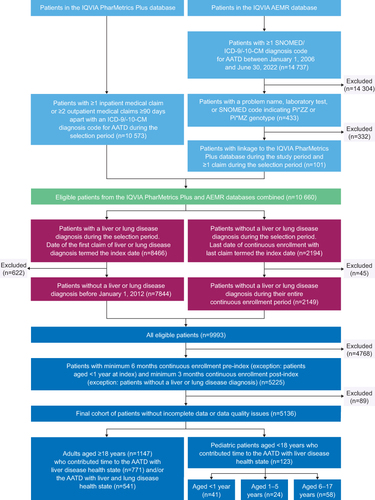
Table 1 Patient Demographics and Characteristics
Figure 2 Liver-Related Clinical Event Rates per Person-Year in (A) Adult Patients (Aged ≥18 Years)a and (B) Pediatric Patientsb. aThe sum of patients in each health state is higher than the number of patients included in the study owing to patients contributing time in more than one health state during the study, according to their disease status. bData are reported for pediatric patients (stratified by age group [<1 year, 1–5 years and 6–17 years]) with AATD with liver disease. Number of events were calculated per person-year (365.25 days); composite data (≥1 liver-related clinical event) are shown for any patient with >1 clinical event.
![Figure 2 Liver-Related Clinical Event Rates per Person-Year in (A) Adult Patients (Aged ≥18 Years)a and (B) Pediatric Patientsb. aThe sum of patients in each health state is higher than the number of patients included in the study owing to patients contributing time in more than one health state during the study, according to their disease status. bData are reported for pediatric patients (stratified by age group [<1 year, 1–5 years and 6–17 years]) with AATD with liver disease. Number of events were calculated per person-year (365.25 days); composite data (≥1 liver-related clinical event) are shown for any patient with >1 clinical event.](/cms/asset/0772d3b5-51c6-4d12-b079-b6de62e26f18/dhme_a_12304573_f0002_c.jpg)
Figure 3 Median Time to First Liver-Related Clinical Event in (A) Adult Patients (Aged ≥18 Years) and (B) Pediatric Patientsa. aData are reported for pediatric patients (stratified by age group [<1 year, 1–5 years and 6–17 years]) with AATD with liver disease. Time to first liver-related clinical event was reported from the index date. Patients with ≥1 clinical event are presented in the composite data.
![Figure 3 Median Time to First Liver-Related Clinical Event in (A) Adult Patients (Aged ≥18 Years) and (B) Pediatric Patientsa. aData are reported for pediatric patients (stratified by age group [<1 year, 1–5 years and 6–17 years]) with AATD with liver disease. Time to first liver-related clinical event was reported from the index date. Patients with ≥1 clinical event are presented in the composite data.](/cms/asset/2219b4f0-17e1-4dc4-a438-970faf3c4adc/dhme_a_12304573_f0003_c.jpg)
Data Sharing Statement
The original de-identified data used in this analysis were obtained from and are the property of IQVIA. IQVIA has restrictions prohibiting the authors from making the data set publicly available. Interested researchers may contact IQVIA to apply to gain access to the study’s data in the same way the authors obtained the data (see https://www.iqvia.com/contact/sf).
