Figures & data
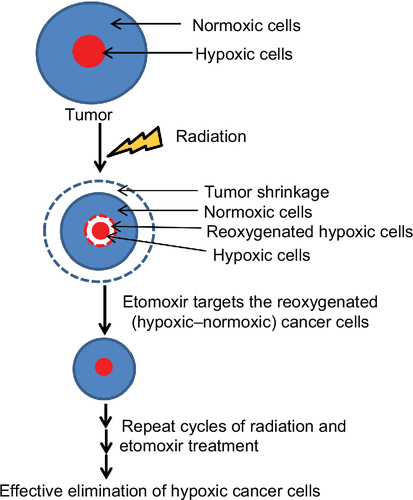
Figure 1 Etomoxir combination improves radiation efficacy against sphere formation by H460 lung epithelial cancer cells.
Abbreviations: Eto, etomoxir; Rad, radiation; SEM, standard error of the mean.
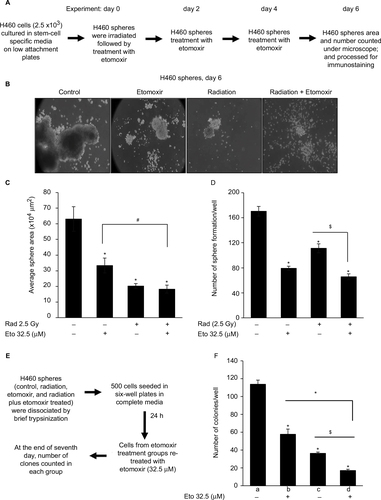
Figure 2 Etomoxir treatment following radiation exposure reduces hypoxic areas in H460 spheres.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; Eto, etomoxir; Rad, radiation; SEM, standard error of the mean.
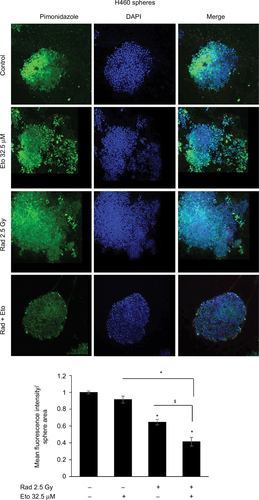
Figure 3 Etomoxir treatment following radiation exposure reduces the expression of proliferation and stemness biomarkers in H460 spheres.
Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; Eto, etomoxir; Rad, radiation; SEM, standard error of the mean.
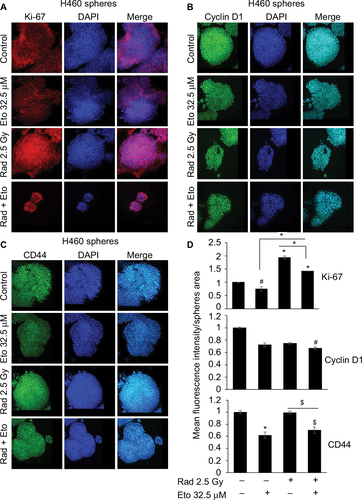
Figure 4 Etomoxir treatment following radiation exposure reduces CPT1 expression in H460 spheres.
Abbreviations: CPT1A, carnitine palmitoyltransferase 1A; DAPI, 4′,6-diamidino-2-phenylindole; Eto, etomoxir; Rad, radiation; SEM, standard error of the mean.
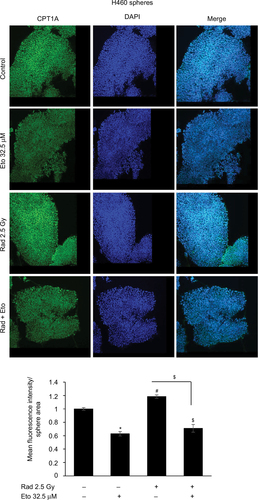
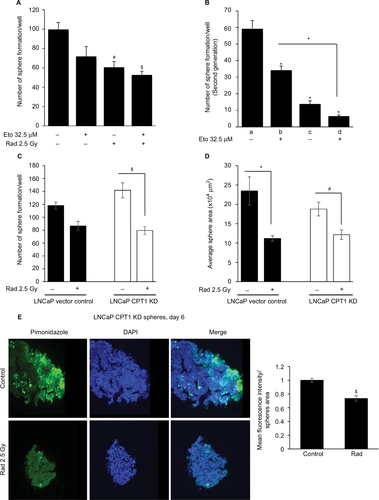
Figure 5 Radiation treatment effectively reduces sphere size and hypoxic areas in CPT1A knockdown LNCaP cells.
Abbreviations: CPT1A, carnitine palmitoyltransferase IA; DAPI, 4′,6-diamidino-2-phenylindole; Eto, etomoxir; KD, knockdown; Rad, radiation; SEM, standard error of the mean.