Figures & data
Table 1 Antibiotic recommendations for treating and preventing invasive meningococcal diseaseCitation31
Table 2 Overview of published clinical studies of MenACWY-CRM in children and adolescents
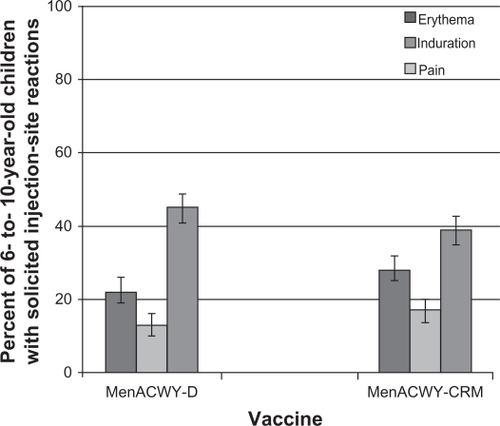
Figure 1 Percent of children aged 2–10 years with seroresponse 30 days following a single dose of MenACWY-D or MenACWY-CRM.
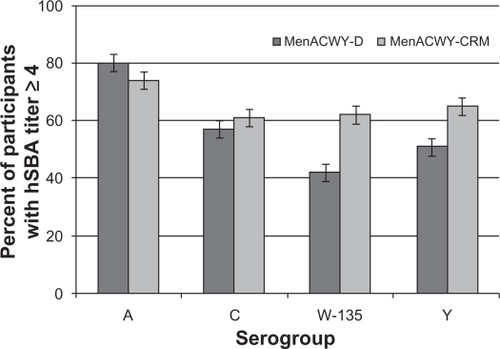
Figure 2 Percent of children aged 2–5 years reporting solicited injection site reactions within the 7 days following a single dose of MenACWY-D (n = 684) or MenACWY-CRM (n = 693).
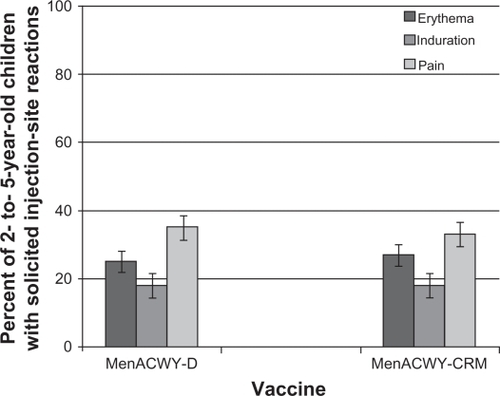
Figure 3 Percent of children 6–10 years reporting solicited injection site reactions within the 7 days following a single dose of MenACWY-D (n = 571) or MenACWY-CRM (n = 582).