Figures & data
Table 1 Demographic data among HIV-1 infected patients with treatment failure (N=359)
Figure 1 Percentage of IAS–USA HIV drug resistance associated mutations and drug resistance by HIVdb program of the Stanford University among 290 HIV-1 infected patients with virologic failure, 2009–2014.
Note: A high of 75.5% of patients had drug resistance to any of the three classes of ART and 86.6% of the patients harbored any of the drug resistance associated mutations.
Abbreviations: ART, antiretroviral therapy; HIV, human immunodeficiency virus; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
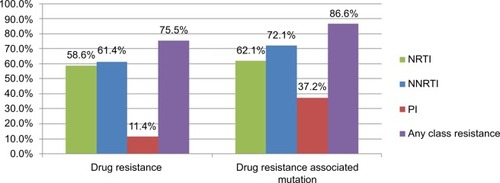
Figure 2 The prevalence of drug resistance to NRTI, NNRTI, and PI among 290 HIV-1 infected patients with virologic failure.
Note: Only 5.9% of the patients had drug resistance to tenofovir. The NNRTI cross-resistance was common and 51.7% of patients were also resistant to rilpivirine although they had no previous exposure to this new second-generation NNRTI.
Abbreviations: HIV, human immunodeficiency virus; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
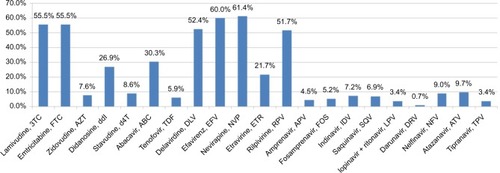
Figure 3 Percentage of ISA–USA HIV drug resistance associated mutations in NRTI, NNRTI, and PI among 290 HIV-1 infected patients with virologic failure.
Notes: The most common NRTI drug resistance associated mutations were M184V (52.1%) and L74V (13.8%). For NNRTI, the most common drug resistance associated mutations were K103N (26.6%) and Y181C, while for PI, these were A71V (13.1%) and L10I (12.4%).
Abbreviations: HIV, human immunodeficiency virus; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
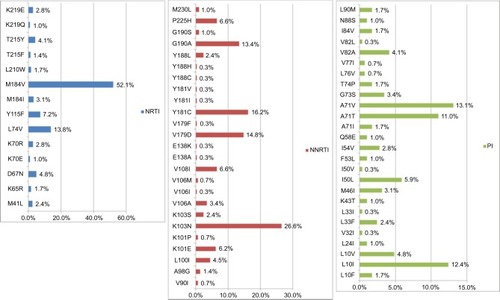
Table 2 Risk factors associated with HIV-1 drug resistance in univariate analysis
Table 3 Risk factors associated with drug resistance in Cox regression model
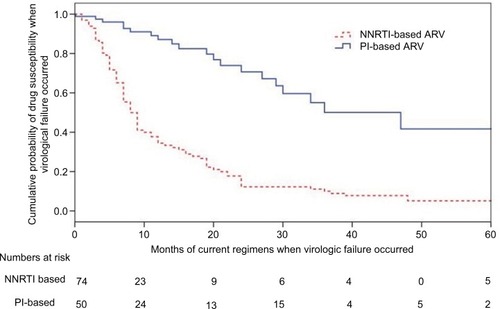
Figure 4 Kaplan–Meier curves for probabilities of developing drug resistances in patients after failure of their current regimen (p<0.0001, log rank test).
Notes: Patients with NNRTI-based regimen were more likely to develop virologic failure, compared to those on PI-based regimens (odds ratio: 4.04, CI: 2.47–6.59; p<0.001). The numbers used in the category at risk were 234. However, 290 samples were successfully tested for resistance. The detailed information for the drug prescription was not available in 56 subjects.
Abbreviations: NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.