Figures & data
Figure 1 Infection cycle of toxigenic Clostridium difficile in the human gastrointestinal track. As C. difficile is an obligate anaerobic bacterium, transmission occurs primarily via spores. Three sources of infection (health care, animal and community residences) are indicated. Spores and some vegetative cells (most of which are eliminated in the hosts stomach) are ingested. Once past the stomach a range of metabolic factors (primary to secondary bile acid ratio, short chain fatty acids) encourages spore germination in the duodenum. After germination, the cells disseminate to the anaerobic folds of the ileum and cecum, forming colonies (assuming dysbiosis). Once in the colon, some cells enter sporulation, others produce toxins. As toxin levels increase, the epithelial barrier is challenged, this in turn initiates the inflammatory response and upregulates the production of anti-toxin antibodies in the host.
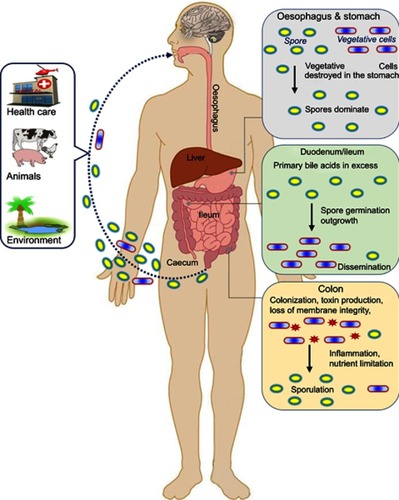
Figure 2 Schematic representation of the toxin genes and regulatory proteins. (A) Pathogenicity locus (PaLoc) region containing the following genes: tcdR, tcdB, tcdE, tcdC and tcdA. The arrows indicate the direction of transcription. TcdC negatively regulates AB toxin expression. Other regulators Sigma D (SigD), the nutritional repressor CodY (known as GTP-sensing transcriptional pleiotropic repressor CodY), catabolite control protein A (CcpA), Stage 0 sporulation protein A (Spo0A) and quorum sensing (QS)) that affect toxin gene transcription (boxed) mostly act via expression of the tcdR gene. (B) Schematic of the binary toxin locus (CdtLoc) and flanking regions with regulatory interactions. CtdR positively regulates the transcription of cdtA and cdtB. CtdR also regulates the production of AB toxins in various 027 strains but not in ribotypes 078 and 012.
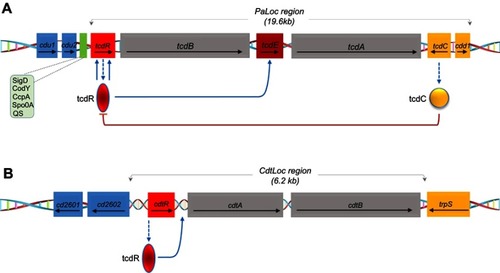
Table 1 Immune-based treatment and prevention of Clostridium difficile infection
Figure 3 Cladogram plots were generated in Galaxy to visualize significantly enriched fungal taxa identified in Clostridium difficile infection (CDI) and non-CDI samples considering each treatment cohort separately (A, untreated; B, fidaxomicin; C, metronidazole; D, vancomycin).Citation131
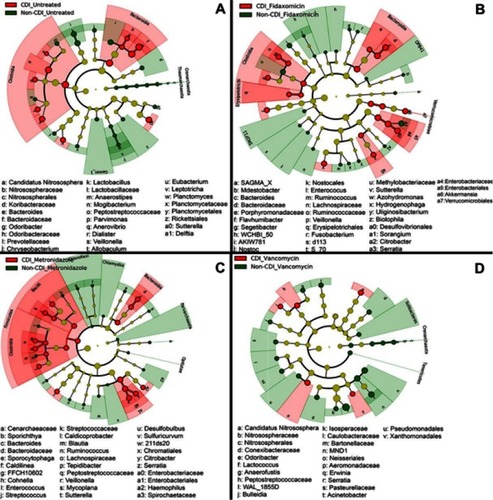
Table 2 Characteristics of some recent studies concerning fecal microbiota transplantation in C. difficile treatment
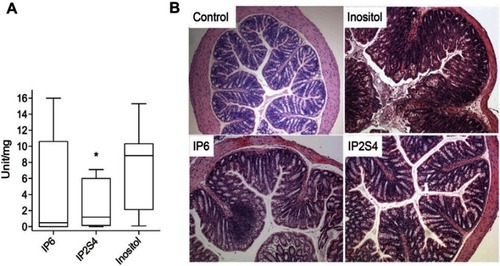
Figure 4 Swiss mice infected with fecal slur from a patient with recurrent Clostridium difficile infection. (A) Oral administration of IP2S4 but not IP6 significantly reduced the acute inflammatory component of colitis compared with administration of myo-inositol. (B) Histological sections of excised colons. Inositol-treated mice (negative control) displayed overt colonic structural changes characterized by mucosal ulceration and overlying exudate, marked acute and chronic inflammatory infiltrate and submucosal edema. IP2S4- and IP6-treated mice had decreased mucosal damage and inflammatory infiltrate. Copyright ©2018. Reproduced with permission from Elsevier. Ivarsson ME, Durantie E, Huberli C, et al. Small-molecule allosteric triggers of Clostridium difficile toxin B auto-proteolysis as a AQ3 therapeutic strategy. Cell Chem Biol. 2018;26(1):17–26.e13.Citation175