Figures & data
Table 1 Aspects included in the TDM survey of clinical laboratories in South Korea
Table 2 Characteristics of 112 clinical laboratories in South Korea
Table 3 Current status of drug assays by 62 clinical laboratories with in-house drug assay facilities (numbers of responding laboratories)
Figure 1 Utilization of drug assays by 112 clinical laboratories in South Korea (number of respondents). (A) Whether laboratories provide drug assay services and have in-house drug assay facilities for five antibiotics, (B) reasons for not having in-house drug assay facilities, (C) laboratories considering whether or not to provide therapeutic drug monitoring (TDM) consulting service in the future, and (D) reasons why therapeutic drug monitoring consulting services are not provided and/or will not be provided.
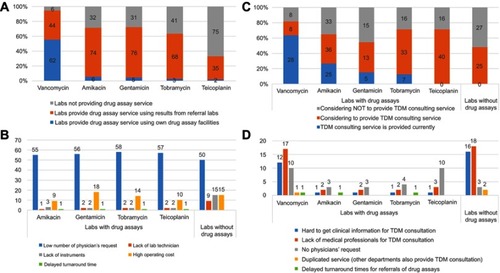
Table 4 Characteristics of drug assay results reporting and TDM consulting services by 62 clinical laboratories (both with and without in-house drug assay facilities, numbers of responding laboratories)
Figure 2 Target concentrations for therapeutic drug monitoring of five antibiotics provided by clinical laboratories to physicians: (A) vancomycin, (B) amikacin, (C) gentamicin, (D) tobramycin, and (E) teicoplanin. The Y-axis represents target concentration (ug/mL). The X-axis represents number of respondents (laboratories). No clinical laboratories provided target peak concentrations for teicoplanin.
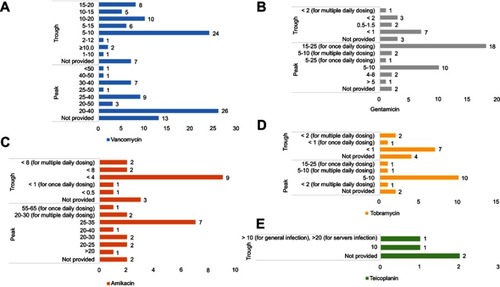
Figure 3 Expectations for other platform-based drug assays, such as point-of-care or smaller scale devices (number of respondents). (A) Needs for other platform-based drug assays, (B) consideration of future installation of other platform-based drug assays, and (C) reasons for needs expressed regarding other platform-based drug assays.
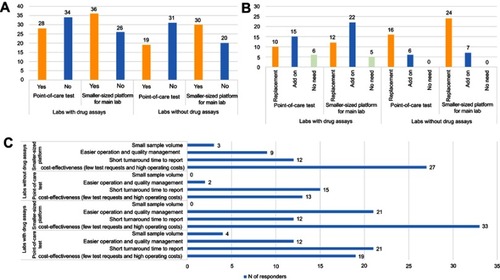
Figure 4 Expectations of the requirements for using other platform-based drug assays, such as (A) point-of-care and (B) smaller scale devices.
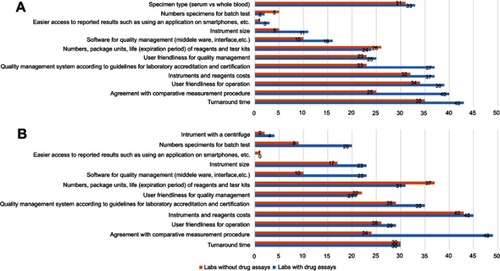
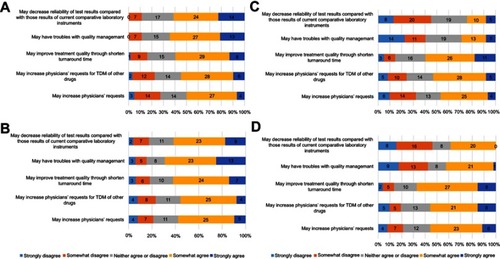
Figure 5 Concerns of the impacts of other platform-based drug assays. (A) Responses from laboratories with in-house drug assays (concerns of the impacts of point-of-care tests), (B) responses from laboratories with in-house drug assays (concerns of the impacts of smaller scale devices), (C) responses from laboratories without in-house drug assays (concerns of the impacts of point-of-care tests), and (D) responses from laboratories without in-house drug assays (concerns of the impacts of smaller scale devices).