Figures & data
Table 1 Primers Used for Amplification of Open Reading Frame Sequences of the OprI and PcrV
Figure 1 (A) Expressed and purified chimeric protein in the SDS-PAGE. The gel (12% w/v) was stained with the Coomassie blue G-250. Lane 2, pellet of the uninduced bacteria with IPTG; lane 1, standard protein size marker (kDa); lane 3, pellet of IPTG-induced bacteria; lane 4, Purification of the chimeric protein on the SDS-PAGE. (B) Confirmation of the chimeric protein through the Western blotting. (C) The OprF protein purified in the SDS-PAGE (12% w/v gel). (D) The OprF protein approved by the Western blotting. (E) The OprI protein purified in the SDS-PAGE by 9% w/v gel. (F) Confirmation of the OprI protein through the Western blotting. (G) The PcrV protein purified in the SDS-PAGE by 12% w/v gel. (H) The PcrV protein approved by the Western blotting.
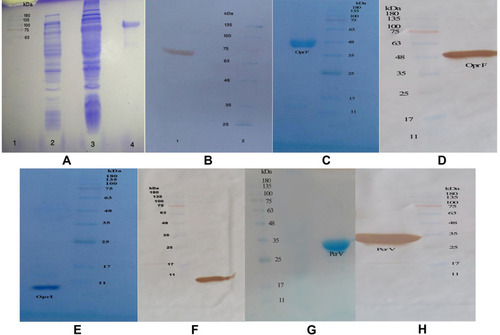
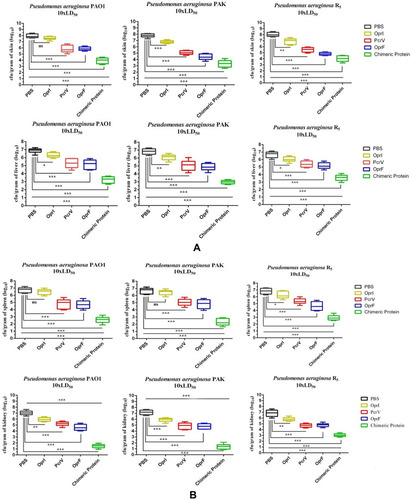
Figure 2 ELISA assay. (A) Total IgG level in the mice immunized with the chimeric and recombinant proteins at different times. (B) The Total IgG bar chart after administration of the second booster in serial dilution antibody for chimeric and recombinant proteins. (C) The binding power of specific antibodies to the Pseudomonas aeruginosa PAO1 whole-cell in the chimeric and recombinant proteins. (D) Comparison of the whole-cell ELISA results between two reference strains of Pseudomonas aeruginosa. *p< 0.05, **p< 0.01 and ***p< 0.001.
Abbreviation: Ns, not significant.
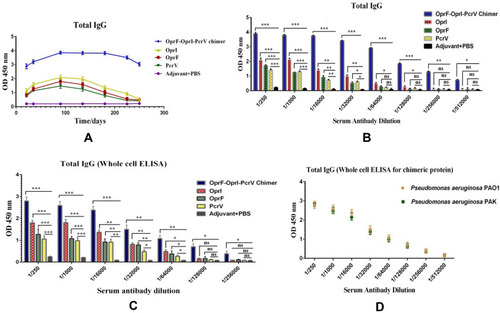
Table 2 The Amounts of Inoculation Doses of Death by the Pseudomonas aeruginosa Strains of PAO1, PAK, and R5 (in the Burned Mice)
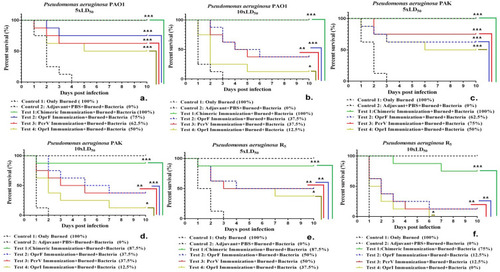
Figure 3 Comparison of survival rate in the two control groups (including non-immune mice that were only burned and those received adjuvant with PBS) and the mice immunized with four vaccine candidates (chimeric protein, OprI, OprF, and PcrV) that were challenged with burn wound infections by the P. aeruginosa strains of PAO1, PAK, and R5. *p< 0.05, **p< 0.01 and ***p< 0.001.
Abbreviation: ns, not significant.