Figures & data
Table 1 MICs of K. pneumoniae Against Tested Agents
Figure 1 The prevalence of carbapenem-resistant or susceptible K. pneumoniae among patients in short-stay ICU patients versus long-term ICU patients. Solid dots represent the length of ICU stay for each isolate, and error bars representing 95% confidence intervals. The chi-square analysis showed short-stay ICU patients was tended to be infected with carbapenem-susceptible K. pneumoniae BSIs (P<0.0001).
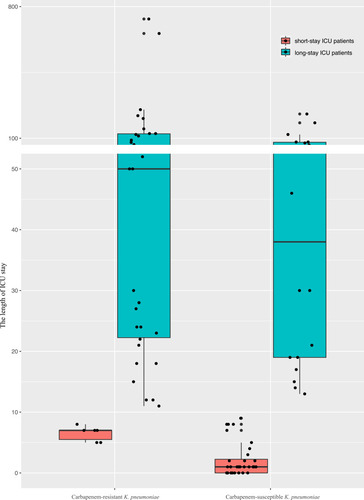
Figure 2 Probability of target attainment (PTA) of 50% or 100% fT MIC for the piperacillin dosing regimens of 2g q6h, 4g q12h, 4g q8h and 4g q6h in critically ill virtual patients with the CRRT intensity of 35–45 mL/kg/h. II, intermittent infusion as 0.5-h infusion; EI, extended infusion as 4-h infusion.
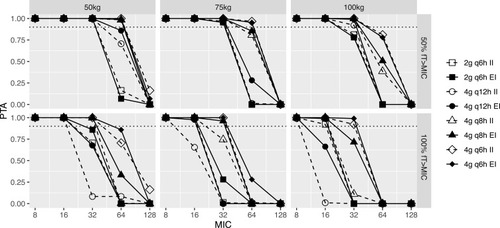
Figure 3 Probability of target attainment of 40% or 100% fT MIC for the imipenem dosing regimens of 0.5g q8h, 0.5g q6h, 1g q8h and 1 g q6h in critically ill virtual patients with the CRRT intensity of 20 mL/kg/h (top) and 40 mL/kg/h (bottom). II, intermittent infusion as 0.5-h infusion.
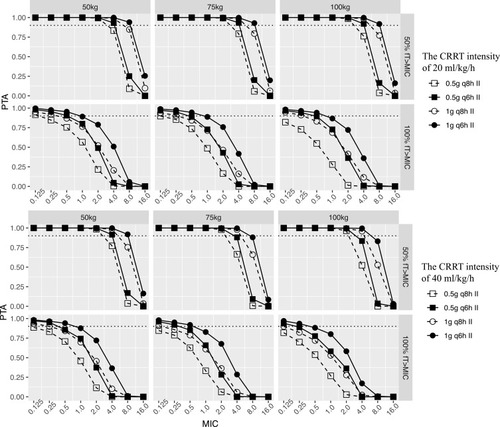
Figure 4 Probability of target attainment (PTA) for a MIC of 64 mg/L when efficacy is defined as 100% fT>MIC and toxicity is defined as an Cmin > 150 mg/L for doses administered to critically-ill patients during CRRT: (A) dosing schedule for critically-ill patients weighing 50 kg in CRRT intensity of 35–45 mL/kg/h; (B) dosing schedule for critically-ill patients weighing 75 kg in CRRT intensity of 35–45 mL/kg/h; (C) dosing schedule for critically-ill patients weighing 100 kg in CRRT intensity of 35–45 mL/kg/h.
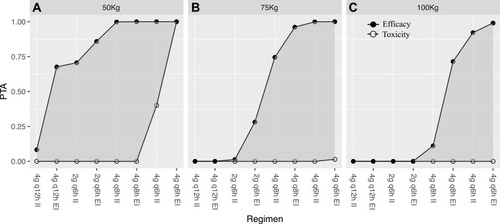
Figure 5 Probability of target attainment (PTA) for a MIC of 1 mg/L when efficacy is defined as 100% fT>MIC and toxicity is defined as an Cmin > 10 mg/L for imipenem doses administered to critically-ill patients during CRRT: (A) dosing schedule for critically-ill patients weighing 50 kg in CRRT intensity of 20 mL/kg/h; (B) dosing schedule for critically-ill patients weighing 50 kg in CRRT intensity of 40 mL/kg/h; (C) dosing schedule for critically-ill patients weighing 75 kg in CRRT intensity of 20 mL/kg/h; (D) dosing schedule for critically-ill patients weighing 75 kg in CRRT intensity of 40 mL/kg/h; (E) dosing schedule for critically-ill patients weighing 100 kg in CRRT intensity of 20 mL/kg/h; (F) dosing schedule for critically-ill patients weighing 75 kg in CRRT intensity of 40 mL/kg/h.
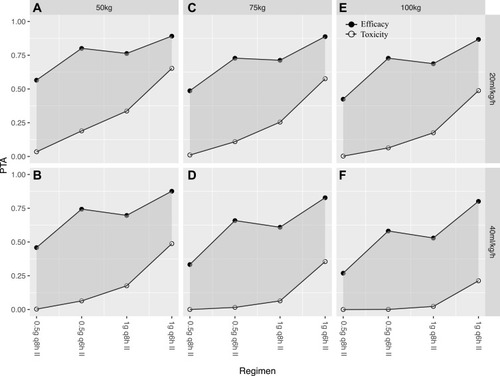
Table 2 The Simulated Cmin of PT and IPM Dosing Regimens in Critically-Ill Patients Requiring CRRT
Table 3 Cumulative Fraction of Response (CFR) to IPM and PT Against the Tested K. pneumoniae Isolated from ICU Patients
