Figures & data
Figure 1 Schematic diagram of adverse drug events selection from Vigibase data used to filter the records.
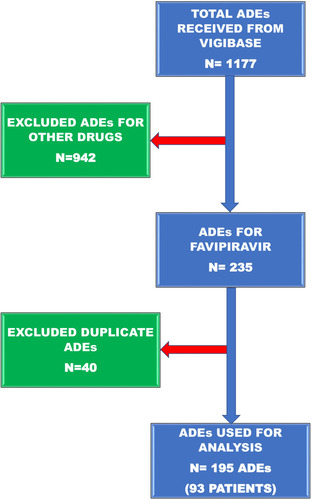
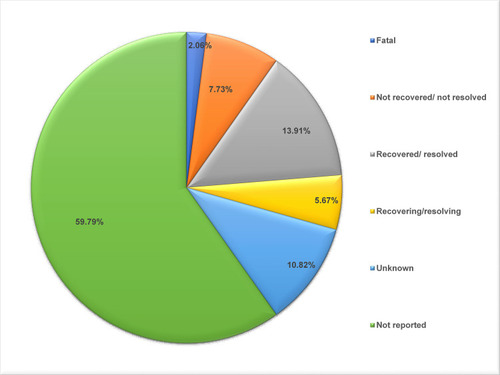
Table 1 Characteristics of Adverse Drug Events (194 ADEs Reported from 93 Individuals) Reported for Favipiravir in WHO Database
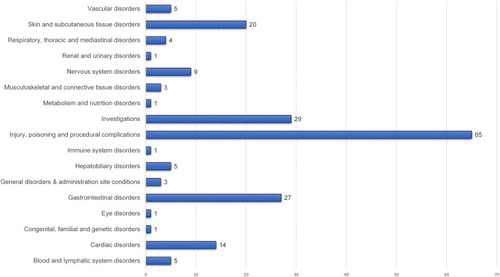
Table 2 Adverse Drug Events Suspected to Be Caused by Favipiravir, as Reported in the WHO Database (N=93)
Figure 2 Distribution of Adverse Drug events reported with Favipiravir use in COVID-19 across continents in Vigibase.
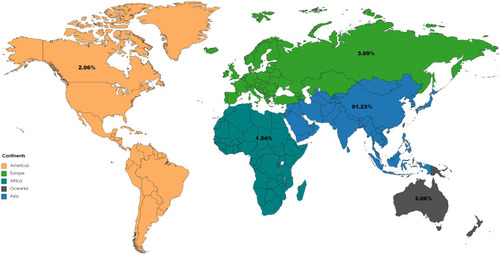
Table 3 Distribution of Characteristics of Different Adverse Drug Events Suspected to Be Caused by Favipiravir Between Age <64 and >64 Years
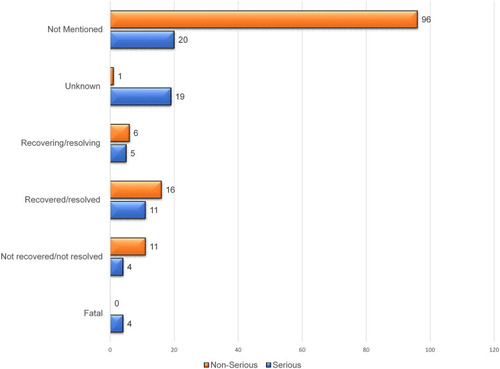
Table 4 Comparison of Serious and Non-Serious Adverse Drug Events Suspected to Be Caused by Favipiravir Among Various Study Characteristics (N = 194)