Figures & data
Figure 1 Schematic flow of the study design. Acute hepatitis symptomatic patients admitted to Assiut University hospitals were assessed for LFTs including liver transaminases and bilirubin. Patients with abnormal LFTs were screened for routine viral hepatitis markers (HAV, HBV, HCV, CMV, and EBV), autoimmune hepatitis markers. Medical history for the patients excluded the possibility of DILI or alcohol-induced liver injury. Samples tested negative to the previously mentioned markers were screened for HEV markers. Patients with liver disease of known etiology (for example HBV, HCV, autoimmune hepatitis, and DILI were not included in this study.
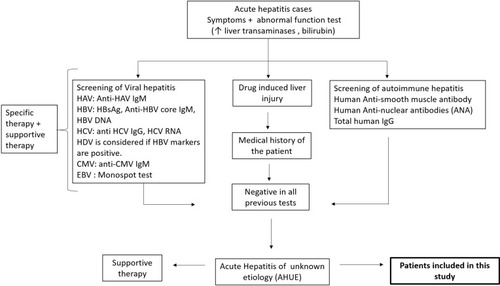
Figure 2 Assessment of HEV markers in AHUE and the outcomes of infection. AHUE (n=300) were screened for HEV markers (anti-HEV IgM, anti-HEV IgG, and HEV RNA). 30 samples were reactive to HEV markers. Four samples were positive to the tested HEV markers (Group 1), 3 samples were positive to anti-HEV IgG and HEV RNA (Group 5) and 2 samples tested positive for anti-HEV IgG (group 6). All the previous patients (Group 1, 5 and 6) recovered without complications. 8 samples were positive to anti-HEV IgM and HEV RNA, and negative for anti-HEV IgG (Group 2), 4 samples tested positive to anti-HEV IgM and anti-HEV IgG, and negative for HEV RNA (Group 3), and 9 samples were tested positive for anti-HEV IgM (Group 4). The previous patients (Group 2, 3, 4, n= 21), 17 patients recovered without complications and 4 patients progressed to FHF. Groups 4 and 6 were also positive for HEV Ag.
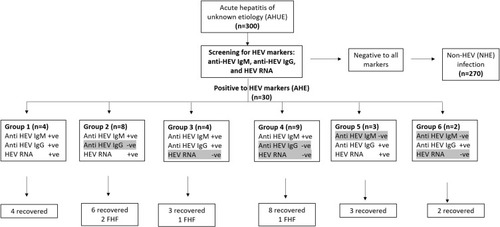
Table 1 Demographic and Laboratory of AHE and NHE Infected Patients
Table 2 Baseline Characteristics of Patients Developed Fulminant Hepatic Failure (FHF)
Table 3 Comparison of Characteristics of FHF Patients and Acute Self-Limited HEV Patients
Figure 3 Comparison of LFTs and HEV markers in FHF patients and self-limited AHE patients. FHF patients (n=4) and self-limited AHE patients (n=26) were assessed for LFTs and HEV markers. (A) The LFTs (ALT, AST, and ALP) were measured in FHF patients and self-limited AHE patients. (B) The absorbance of anti-HEV IgM (A450/C.O.) was compared in FHF patients and self-limited AHE patients. *, ** means p<0.05 and p≤ 0.01, respectively as determined by two-tailed student’s t test.
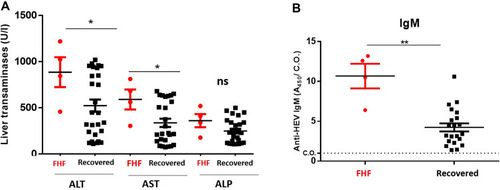
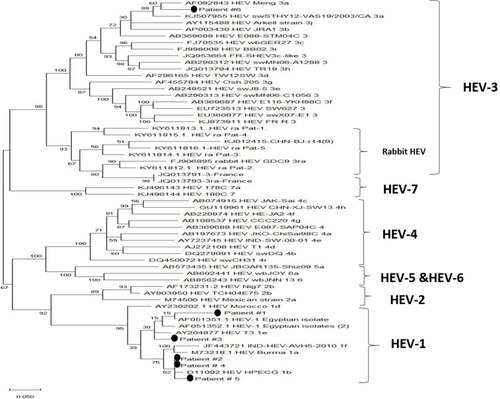
Figure 4 Phylogenetic analysis of the isolated viruses. A maximum-likelihood phylogenetic tree was constructed using various HEV reference sequences with 1000 bootstrap replicas. GenBank accession numbers are shown for each HEV reference strain used in the phylogenetic analysis. The isolated viruses from the patients were remarked it by putting a circle beside them.