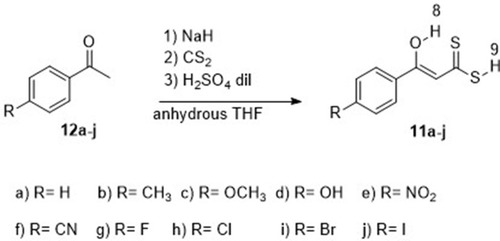
Figures & data
Table 1 Anti-M. tuberculosis Effects and Cytotoxicity Levels of Compounds 11a–j
Table 2 REMA-Determined MIC100 Values of 11e Against Virulent, Non-Virulent and RIF-Resistant M. tuberculosis Strains
Table 3 Calculated Physicochemical Properties of Compounds 11a-j
Table 4 Predicted Toxicities of the Synthesized Compounds 11a-j
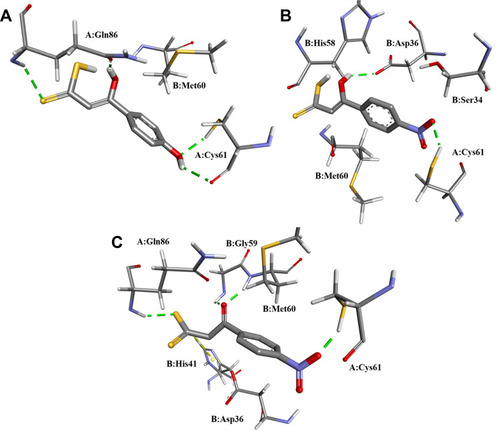
Table 5 Calculated Binding Free Energy (ΔG) from Molecular Docking Results of the Two Tautomeric Forms (Keto and Enol) of 11a-j Compounds Within HadAB Active Site