Figures & data
Table 1 Primer and Probe Sequences Used for Real-Time Reverse Transcription-Polymerase Chain Reaction (PCR) to Detect Severe Acute Respiratory Syndrome Coronavirus 2
Table 2 Limit of Detection of LabTurboTM AIO COVID-19 RNA Testing Kit for N1 and E Using the Rotor-Gene-Q Real-Time PCR Instrument
Table 3 Cross-Reaction of the LabTurboTM AIO COVID-19 RNA Testing Assay with Known Respiratory Viruses in Clinical Samples or Cell Supernatants
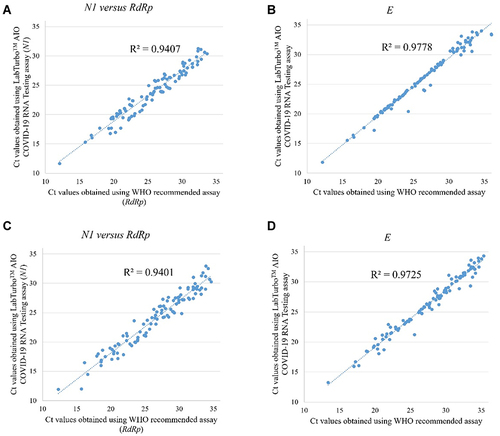
Figure 1 Correlation between results obtained using LabTurboTM AIO COVID-19 RNA testing assay and WHO-recommended assay with the Rotor-Gene-Q real-time PCR instrument. (A) SARS-CoV-2 N1 versus RdRp screening results. (B) SARS-CoV-2 E screening results; correlation between results obtained using LabTurboTM AIO COVID-19 RNA testing assay and WHO-recommended assay with the Roche LightCycler96 instrument. (C) Shows SARS-CoV-2 N1 versus RdRp gene results. (D) Shows SARS-CoV-2 E gene results.