Figures & data
Table 1 The Baseline Characteristics of the Study Subjects
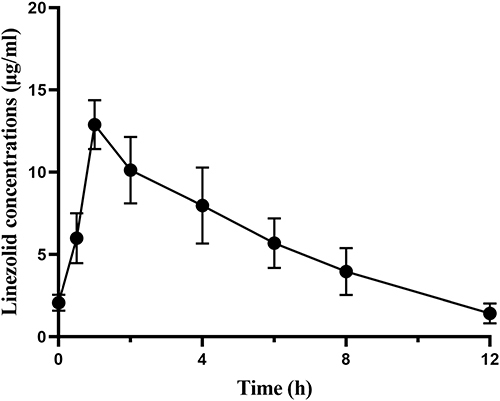
Table 2 The Pharmacokinetic Parameters of Linezolid in Plasma
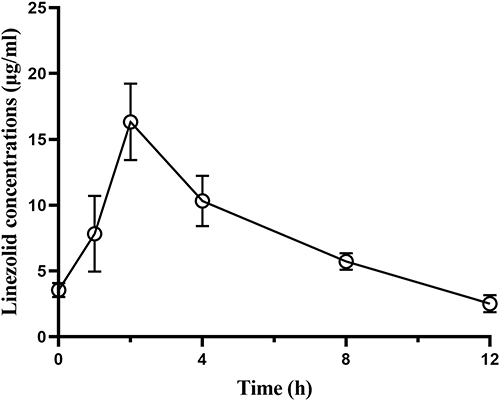
Table 3 The Pharmacokinetic Parameters of Linezolid in the Sputum
Table 4 The PTAs of Linezolid in Plasma and Sputum Samples for Different Minimum Inhibitory Concentrations
Table 5 Minimum Inhibitory Concentration Values and Distribution Frequency of Linezolid Against Gram Positive Cocci (Data Obtained from the European Committee on Antimicrobial Susceptibility Testing, EUCAST)
Table 6 The CFR Values for Different Dosing Regimens Against MRSA, Enterococcus faecium, Enterococcus faecalis, and Streptococcus pneumoniae in Plasma and Sputum