Figures & data
Table 1 Demographic Data of the 107 HIV-1-Infected Patients with NNRTI-Based STR Treatment Failure
Table 2 Demographic, Treatment and Resistance Differences Between the 29 Patients Who Failed to the Initial STR and 78 Patients with Virological Failure After Switching to STRs in Univariate Analysis
Table 3 Risk Factors Associated with Doravirine Resistance in Univariate Analysis
Figure 1 Rates of mutations associated with resistance to INSTIs, PIs, NNRTIs and NRTIs among the 107 enrolled patients with HIV-1 infection and virological failure to STRs. Overall, 88% of the patients had mutations associated with drug resistance to any of the four classes of antiretroviral drugs, including 88% with resistance to NNRTIs, 76% to NRTIs, 5% to PIs and 2% to INSTIs.
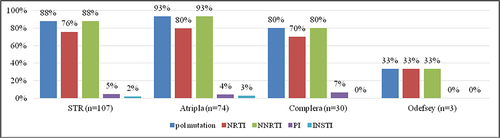
Figure 2 The prevalence of mutations associated with drug resistance to INSTIs, PIs, NNRTIs and NRTIs among the 107 enrolled patients with HIV-1 infection and virological failure to STRs. The most common mutations associated with resistance to NRTIs were M184V (57.9%) and K65R (20.6%); for NNRTIs K103N (35.5%), V179D (20.6%) and L100I (15%); for PIs L33F (1.9%), K20T (1.9%) and V32I (1.9%), and for INSTIs G148H (0.9%) and G140S (0.9%).
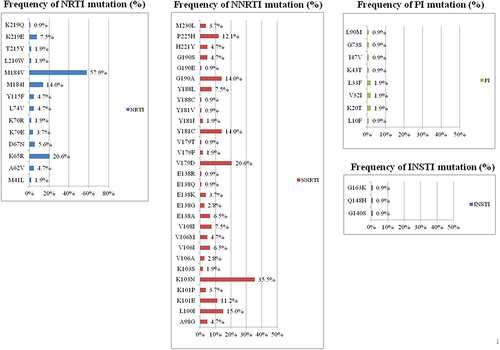
Figure 3 Differences in prevalence of mutation point between 29 patients who failed to the initial STRs and 78 patients with failure after switching to STRs.
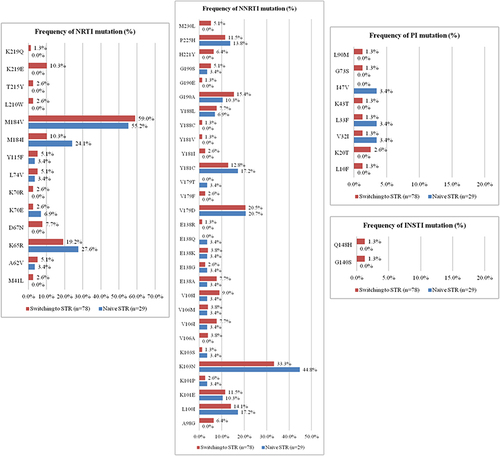
Figure 4 The prevalence of doravirine resistance-associated mutations are shown according to the IAS-USA 2019 HIV drug resistance-associated mutation list. There were no statistically significant differences in the percentage of specific doravirine resistance-associated mutations between the 29 patients who failed to the initial STRs and 78 treatment failures after switching to STRs.
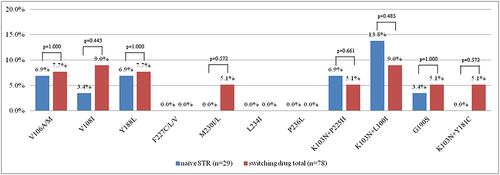
Figure 5 Prevalence rates of resistance to INSTIs, PIs, NNRTIs and NRTIs among the 107 enrolled patients with HIV-1 infection and virological failure to STRs. Overall, 88% of the patients had drug resistance to any of the four classes of antiretroviral drugs, including 86% who had resistance to NNRTIs, 76% to NRTIs, 3% to PIs and 2% to INSTIs.
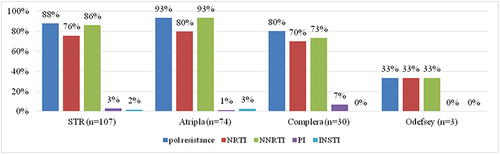
Figure 6 The prevalence of drug resistance to NRTIs, NNRTIs, PIs and INSTIs among the 107 enrolled patients with HIV-1 infection and virological failure stratified by different NNRTI-based STRs. The figure shows that a total of 28% of 107 patients resistance to tenofovir (TDF), 4% to zidovudine (AZT) and 64% to doravirine.
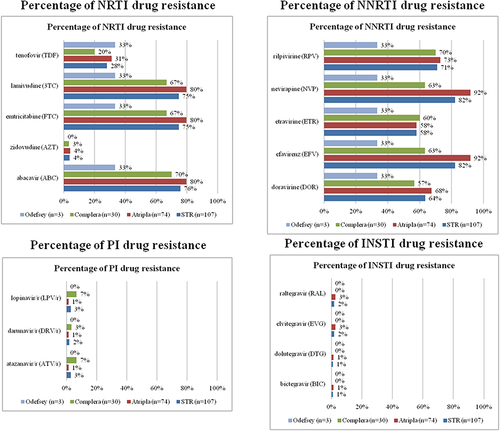
Table 4 Risk Factors Associated with the K65R Mutation in Univariate Analysis
