Figures & data
Table 1 MIC (mg/L) of Antibiotics Against ESBL-Producing E. coli ST405 and MRSA
Figure 2 An overview of synthesis of butyl 2-bromoisonicotinate (3) and its derivatives (5a–5h) via SMC reaction.
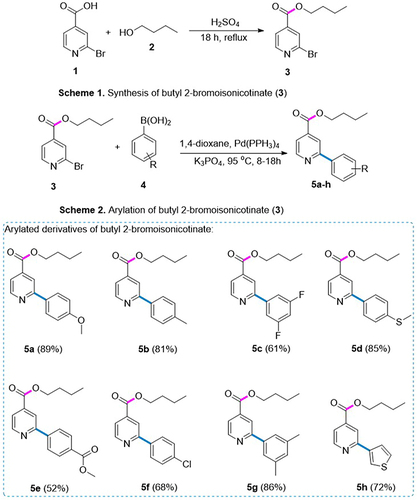
Table 2 Zone of Inhibition (mm) of ESBL-Producing E. coli ST405 Agar Well Diffusion Assay
Table 3 Zone of Inhibition (mm) of MRSA by Agar Well Diffusion Assay
Table 4 MIC (w/v) and MBC (w/v) of Different Molecules Against ESBL-Producing E. coli ST405
Table 5 MIC (w/v) and MBC (w/v) of Different Molecules Against MRSA
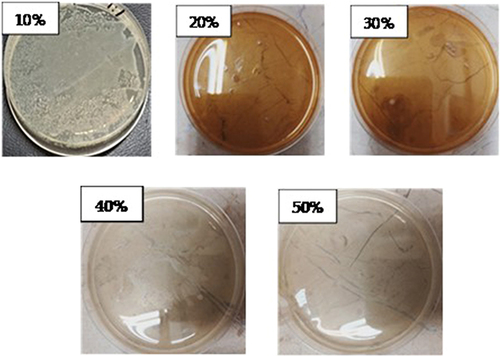
Figure 3 MIC of the ESBL-producing E. coli ST405 and MRSA isolate X-axis are the isolates, and Y-axis is the different concentrations of the molecules, -ve: negative control +ve: positive control.
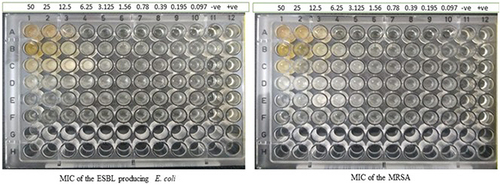
Table 6 Binding Energies and Interaction Pattern of the Parent Molecule (3) and Its Analogues (5a–h)
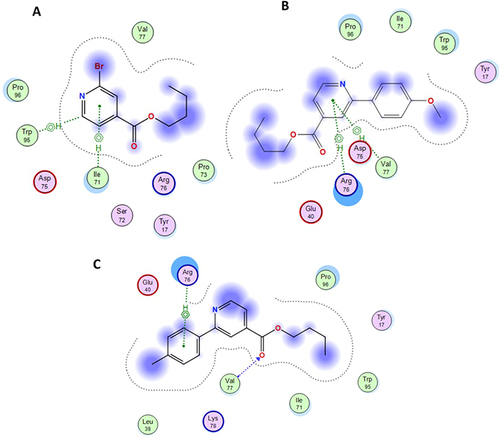
Figure 5 The best and stable conformations of the highly active synthesized molecules (5a and 5b) including parent molecule (3). (A) Molecule (3) forming 2 hydrophobic bonds with Ile71 and Trp95. (B) Molecule (5a) forming 2-hydrophobic bonds with Arg76 and Val77. (C) Molecule (5b) forming 1H bond and 1-hydrophobic bonds with Val77 and Arg76.