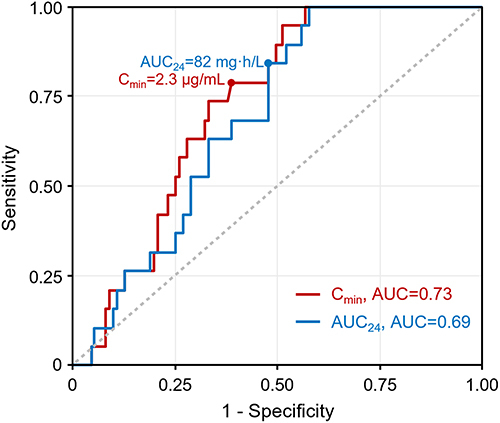
Figures & data
Table 1 Patients Demographics and Clinical Outcomes
Table 2 Univariate and Multivariate Analysis of Clinical Efficacy of Polymyxin B
Table 3 Univariate and Multivariate Analysis of 30-Day All-Cause Mortality
Figure 1 Kaplan–Meier survival estimates for 30-day all-cause mortality.
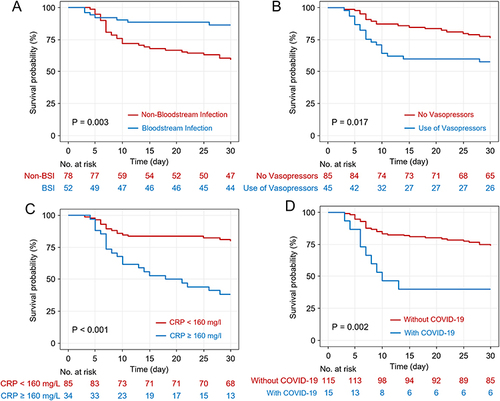
Table 4 Analysis of Factors Influencing Acute Kidney Injury During Polymyxin B Treatment