Figures & data
Figure 1 Gastric proton pump H+/K+ ATPase and its functions. The gastric H+/K+ ATPase mediates the transport of K+ ions in the extracellular fluid into the cell, while intracellular H+ ions are simultaneously pumped out of the cell against a high concentration gradient to complete ion transport and gastric acid secretion. H+/K+ ATPase activity is stimulated by inflammatory and other factors by the bacterium Helicobacter pylori, leading to excessive gastric acid secretion and GERD. This is the mechanism targeted by PPI therapy. H+/K+ ATPases contain the α-subunit and β-subunit. The α-subunit is made up of 1035 amino acids. ATP-binding, acyl-phosphorylation, inhibitor-binding, and ion-recognition sites are included in the α-subunit. The 290-amino-acid β-subunit is responsible for enzyme assembly and plays a role in enzyme activity.
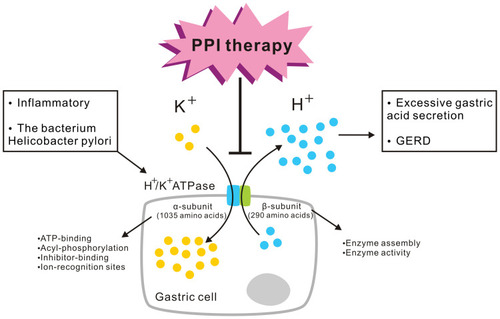
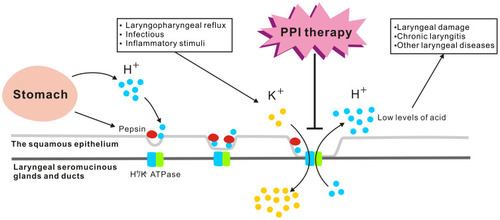
Figure 2 Laryngeal H+/K+ ATPases. The H+/K+ ATPase proton pump is located mainly in the laryngeal seromucinous glands and ducts rather than in the squamous epithelium, which may be responsible for regulating seromucinous secretion in two conditions: (1) direct acid reflux in LPRD, (2) first-stage reflux, when reactivation during subsequent acid exposure leads to pepsin binding and thus to tissue autodigestion, which together with cellular necrosis regulates the pH of the interstitium and may lead to laryngopharyngeal reflux-induced acid exposure. The laryngeal H+/K+ ATPase is activated by laryngopharyngeal reflux and other infectious or inflammatory stimuli. The laryngeal H+/K+ ATPase secretes low levels of acid, which may induce laryngeal damage and, subsequently, chronic laryngitis or other laryngeal diseases. The pathologically acidic environment of the oropharynx in patients without gastroesophageal reflux is effective for PPI therapy, as the larynx is one of the extragastric targets of these drugs.