Figures & data
Table 1 Baseline Characteristics at Inclusion by Group
Figure 1 Head map showing the 7-point ordinal WHO scale across the study period of 24 weeks. Number refers to patients’ identification in the study.
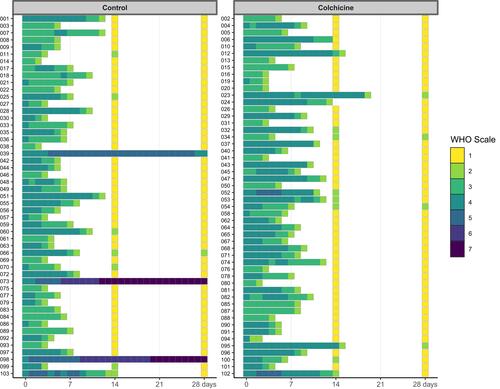
Table 2 Study End-Points by Group