Figures & data
Table 1 Baseline Demographic and Disease Characteristics
Figure 1 Percentage of responders.
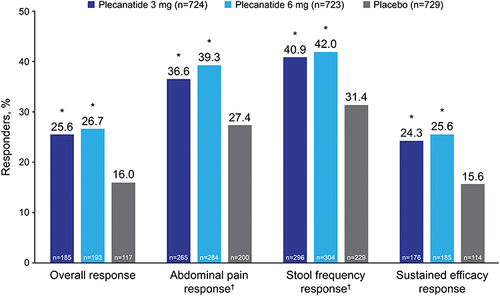
Figure 2 LSM changes from baseline in (A) abdominal pain, (B) abdominal discomfort, (C) abdominal fullness, (D) bloating, and (E) cramping by week during the 12-week treatment period and 2-week posttreatment period.
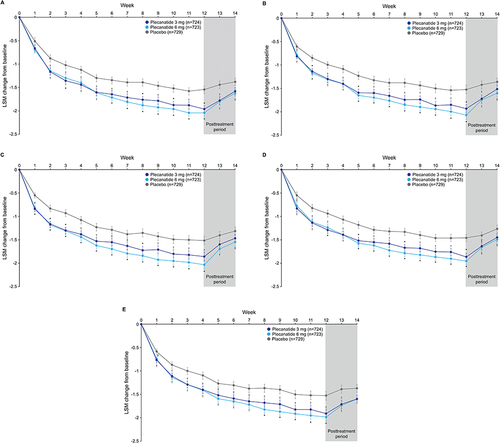
Table 2 Secondary Efficacy Outcomes Across 12 Weeks of Treatment
Table 3 Adverse Events (Safety Population)
