Figures & data
Table 1 Singapore pneumococcal seroprevalence data
Table 2 Effects of pneumonia on the cardiovascular system
Table 3 Serotypes included in pneumococcal vaccines
Figure 1 Functional immune responses for pneumococcal serotype 1 (GMT) in the pivotal noninferiority trial (Study 004) measured pre- and post-vaccination using a functional OPA assay.
Abbreviations: GMT, geometric mean titer; OPA, opsonophagocytic activity; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
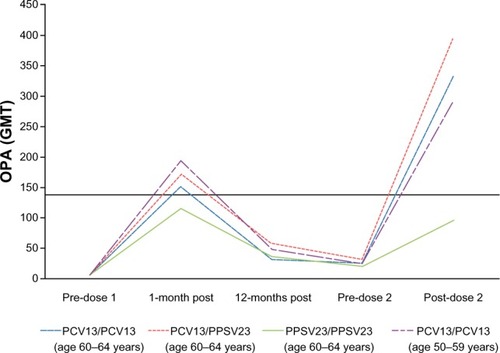
Figure 2 Functional immune responses for pneumococcal serotype 1 (GMT) in the pivotal noninferiority trial (Study 3005) measured pre- and post-vaccination using a functional OPA assay.
Abbreviations: GMT, geometric mean titer; OPA, opsonophagocytic activity; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
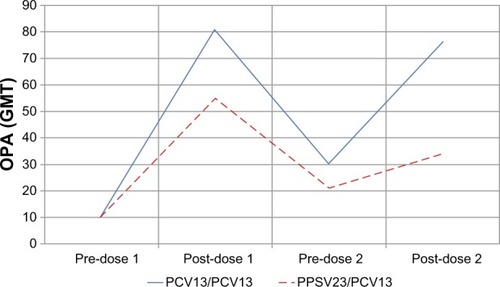
Table 4 Advisory Committee on Immunization Practices 2013 updates/highlights related to pneumococcal vaccination in adults
Table 5 Advisory Committee on Immunization Practices 2012 recommendations on medical conditions or other indications for administration PCV13 and PPSV23 for adults aged ≥19 years,Table Footnote* by risk group
