Figures & data
Figure 1 Patient disposition.
Abbreviations: AE, adverse events; mITT, modified intent-to-treat; qd, once daily; bid, twice daily.
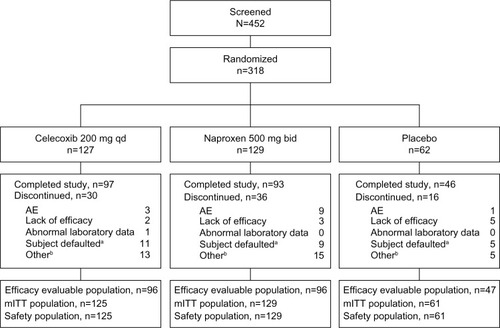
Table 1 Baseline demographic and clinical characteristics
Table 2 Patient’s assessment of arthritis pain (VAS) at week 6 (efficacy evaluable population)
Figure 2 Patient’s Global Assessment of Arthritis: modified intent-to-treat population.
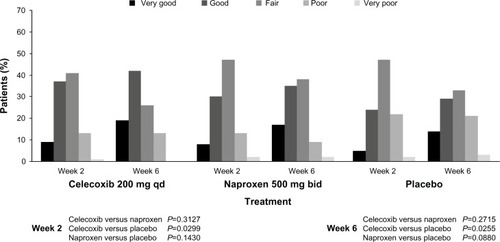
Figure 3 Physician’s Global Assessment of Arthritis: overall ratings (modified intent-to-treat population).
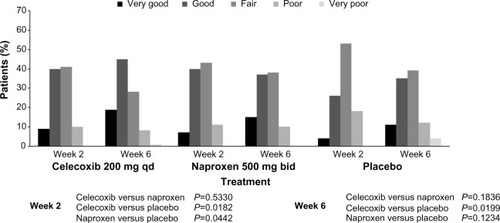
Table 3 WOMAC, upper gastrointestinal tolerability, and APS pain scores
Table 4 Treatment-related adverse events occurring in ≥2% of patients (in decreasing order of occurrence)
