Figures & data
Table 1 Diagnostic criteria for IBSTable Footnote*
Table 2 Differential diagnosis in patients presenting with symptoms of IBS and alarm symptoms/warning signs
Figure 1 Symptom improvement with loperamide.
Abbreviation: IBS-D, diarrhea-predominant irritable bowel syndrome.
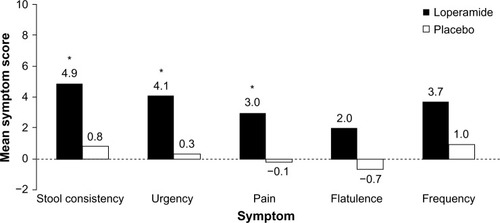
Figure 2 Reduction of IBS symptoms with Bifidobacterium infantis capsule administered once daily vs placebo.
Abbreviations: IBS, irritable bowel syndrome; IBS-D, diarrhea-predominant IBS.
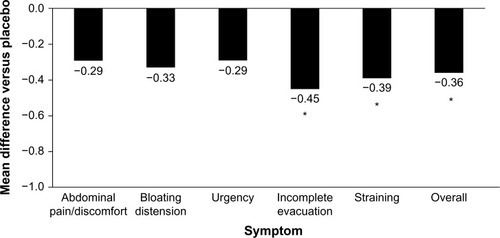
Figure 3 Percentage of patients reporting GI symptoms.
Abbreviations: BSFS, Bristol Stool Form Scale; GI, gastrointestinal; IAC, incompletely absorbed carbohydrates; IBS-D, diarrhea-predominant irritable bowel syndrome.
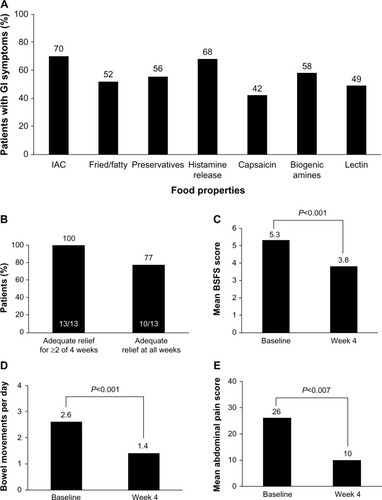
Figure 4 Efficacy of alosetron in females with severe IBS-D.
Abbreviations: IBS, irritable bowel syndrome; IBS-D, diarrhea-predominant IBS.
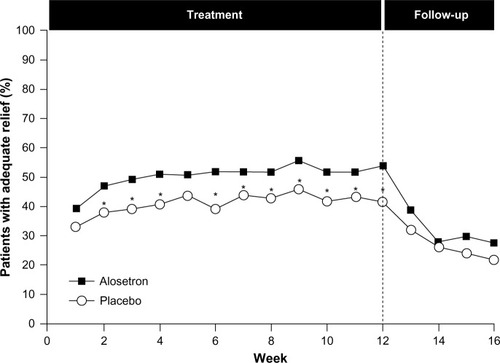
Figure 5 Efficacy of rifaximin for patients with IBS-D.
Abbreviation: IBS, irritable bowel syndrome.
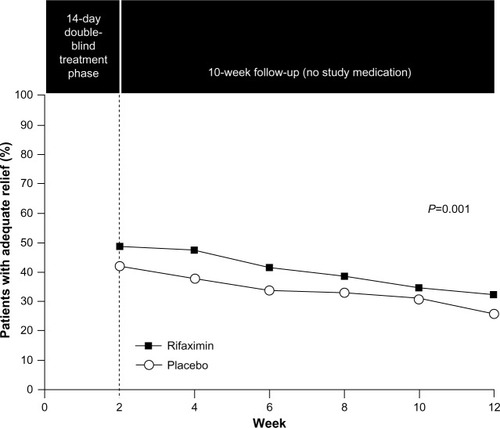
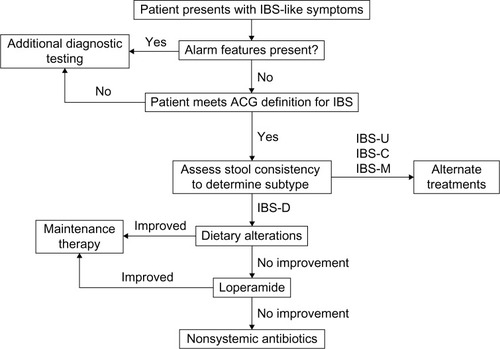
Figure 6 Diagnostic and treatment algorithm for IBS-D.