Figures & data
Table 1 Composition of 734THIF polymeric nanoparticle systems (734N) and blank EE–PVA nanoparticle (blank-N)
Figure 2 The HPLC chromatogram of 734THIF.
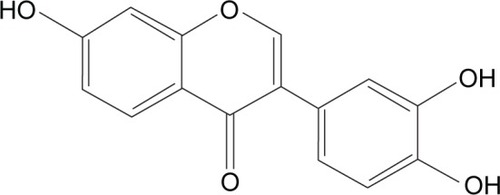
Abbreviations: HPLC, high-performance liquid chromatography; 734THIF, 7,3′,4′-trihydroxyisoflavone.
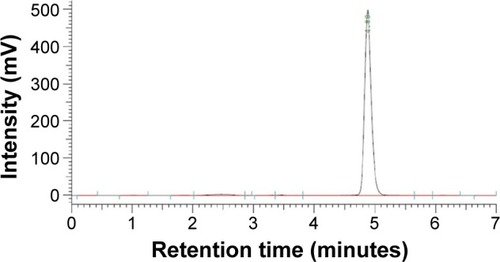
Table 2 The drug loading efficiency, encapsulation efficiency, particle size, and PI of 734THIF polymeric nanoparticle systems
Figure 3 Particle size distribution and morphology of 734THIF nanoparticle formulations.
Notes: Particle size distribution of (A) raw 734THIF, (B) 734THIF:EE:PVA (1:2:2), (C) 734THIF:EE:PVA (1:4:4), (D) 734THIF:EE:PVA (1:8:8). (E) Morphology of 734THIF:EE:PVA (1:8:8) by TEM.
Abbreviations: 734THIF, 7,3′,4′-trihydroxyisoflavone; PVA, polyvinyl alcohol; EE, eudragit E100; TEM, transmission electron microscopy; 734N, 734THIF nanoparticles.
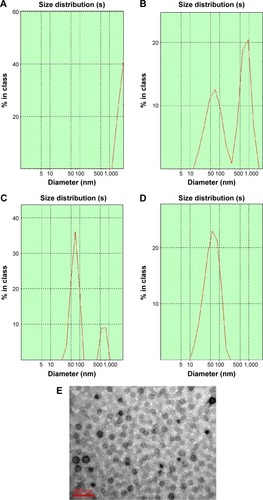
Figure 4 Crystalline-to-amorphous transformation of 734THIF nanoparticle formulation, as demonstrated by (A) powder XRD and (B) DSC.
Abbreviations: 734THIF, 7,3′,4′-trihydroxyisoflavone; XRD, X-ray diffractometry; DSC, differential scanning calorimetry.
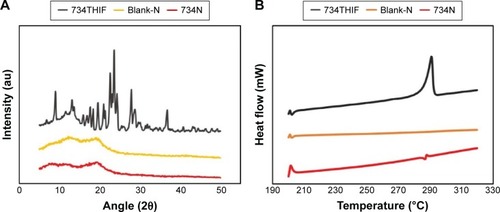
Figure 5 Intermolecular hydrogen-bond formation between EE–PVA polymer and 734THIF, as demonstrated by (A) FTIR and (B) 1H-NMR spectra.
Abbreviations: 734THIF, 7,3′,4′-trihydroxyisoflavone; EE, eudragit E100; PVA, polyvinyl alcohol; FTIR, Fourier transform infrared; 1H-NMR, 1H-nuclear magnetic resonance; ppm, parts per million.
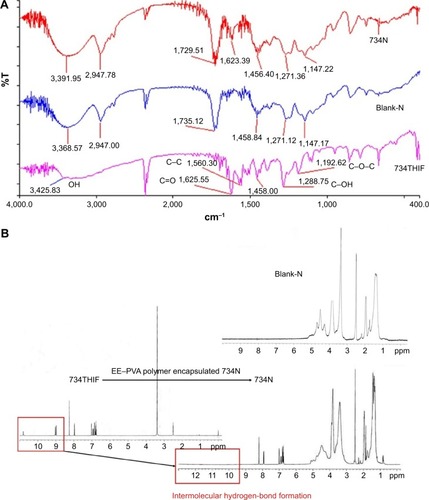
Figure 6 (A) The cell viability of 734THIF nanoparticle formulation. (B) Photographs of 734N dispersed in various pH buffer solutions. The solubility of 734THIF (C) and 734N (D) in 0.2 M KH2PO4 buffer solution at various pH.
Note: *P<0.05 was statistically significant difference compared with 734THIF.
Abbreviations: 734THIF, 7,3′,4′-trihydroxyisoflavone; 734N, 734THIF nanoparticles.
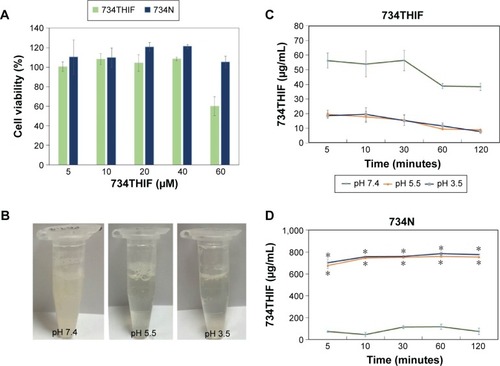
Figure 7 The surface morphology of lyophilized powder 734THIF (A) and 734THIF nanoparticle formulation (B), as shown by SEM.
Abbreviations: 734THIF, 7,3′,4′-trihydroxyisoflavone; SEM, scanning electron microscopy.
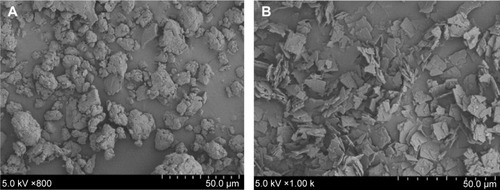
Figure 8 The contents of 734THIF delivered from raw 734THIF and 734N to different skin layers: stratum corneum (A), epidermis (B), and dermis (C).
Notes: Values are mean ± SD (n=5). *P<0.05: statistically significant difference compared with raw 734THIF.
Abbreviations: 734THIF, 7,3′,4′-trihydroxyisoflavone; h, hours; SD, standard deviation; 734N, 734THIF nanoparticles.
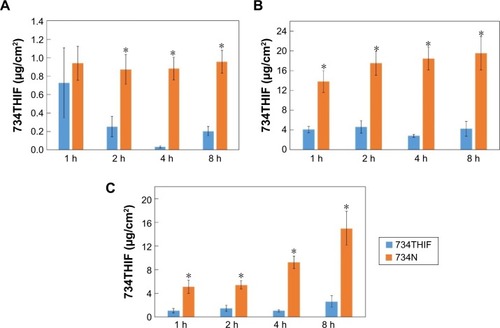
Table 3 The concentration of raw 734THIF and 734 nanoparticle formulation required to scavenge 50% of DPPH free radicals (SC50)
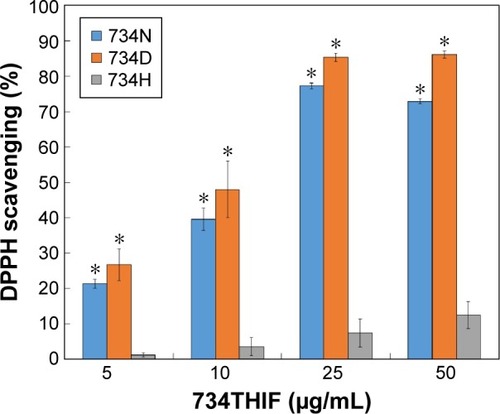
Figure 9 The DPPH radical scavenging activity of raw 734THIF suspended in deionized water (734H) and DMSO (734D), and 734THIF nanoparticle formulation suspended in deionized water (734N).
Notes: Values are mean ± SD (n=3). *P<0.05: statistically significant difference compared with 734H.
Abbreviations: DPPH, 2,2-diphenyl-1-picrylhydrazyl; 734THIF, 7,3′,4′-trihydroxy-isoflavone; DMSO, dimethyl sulfoxide; SD, standard deviation.