Figures & data
Figure 1 Scanning electron micrograph of glutaraldehyde crosslinked nanoparticles prepared at pH 8, 60k resolution. The nanoparticles were found to be of uniform size and narrow size distribution.

Figure 2 Effect of glutaraldehyde concentration on diameter and polydispersity index of noscapine (5 mg/mL) loaded human serum albumin (HSA) nanoparticles prepared with 10 mM NaCl solution and 100 mg of HSA protein at pH 8.
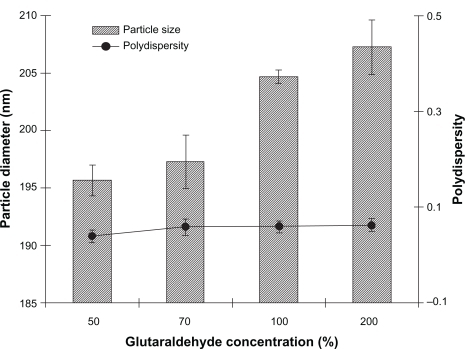
Figure 3 Effect of pH on diameter and polydispersity index of noscapine (5 mg/mL) loaded human serum albumin (HSA) nanoparticles prepared with 10 mM NaCl solution and 100 mg of HSA protein.
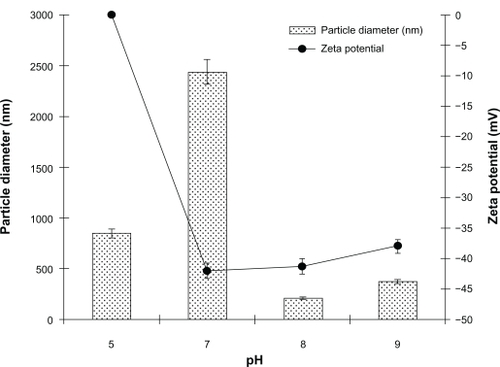
Figure 4 Stability of noscapine (5 mg/mL) loaded human serum albumin (HSA) nanoparticles over 5 days. Particle size was monitored. Noscapine-loaded HSA nanoparticles prepared with 10 mM NaCl solution and 100 mg of HSA protein at pH 8.
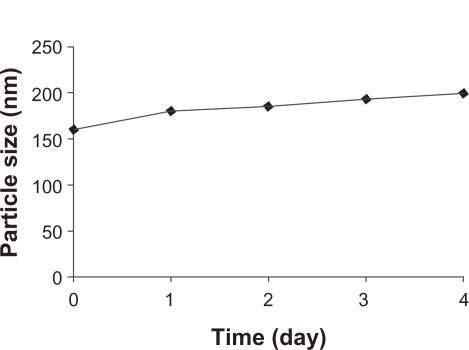
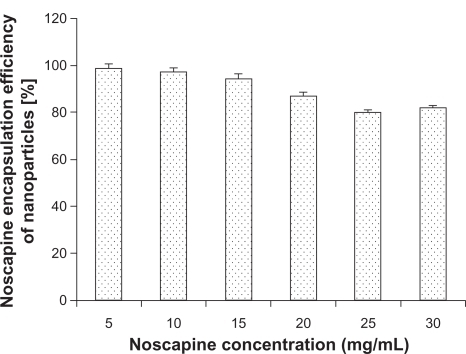
Figure 5 Noscapine encapsulation of human serum albumin nanoparticles (50 mg/mL) in dependence on noscapine concentration.