Figures & data
Figure 1 Preparation scheme of DP7-C-modified AZT-loaded liposomes (AZT-D-LPs) using the thin-film hydration method.
Abbreviations: AZT, azithromycin; AZT-D-LPs, DP7-C-modified AZT-loaded liposomes; Chol, cholesterol; PBS, phosphate-buffered saline; SPC, soybean phosphatidylcholine.
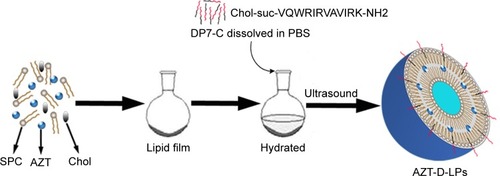
Figure 2 Characterization of AZT-D-LPs.
Notes: (A) Particle size distribution spectrum of AZT-D-LPs. (B) TEM image of AZT-D-LPs. (C) Zeta potential distribution of AZT-LPs. (D) Photographs of free AZT in water (a), nonmodified AZT-LPs (b), D-LPs (c), AZT-D-LPs (d), D-LPs + free AZT (e). (E) In vitro release profile of AZT from free AZT, nonmodified AZT-LPs, and AZT-D-LPs.
Abbreviations: AZT, azithromycin; AZT-D-LPs, DP7-C-modified AZT-loaded LPs; AZT-LPs, AZT-modified liposomes; D-LPs, DP7-C-modified blank LPs; TEM, transmission electron microscope.
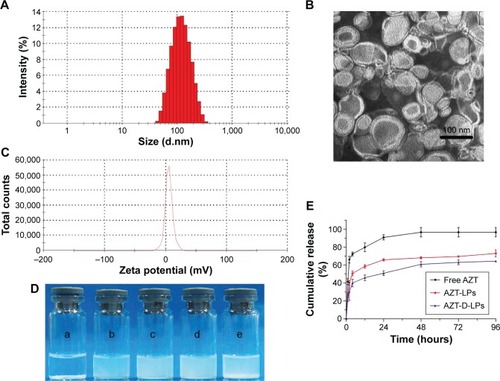
Table 1 In vitro antimicrobial activity of free AZT, AZT-LPs, D-LPs, and AZT-D-LPs against S. aureus and E. coli strains
Figure 3 Efficacy of DP7-C and/or AZT formulations in S. aureus infectious mouse model.
Notes: BALB/c mice were infected with 1×108 CFU/500 μL S. aureus strain 33591 by intraperitoneal injection; antimicrobial drug was administered 1 hour after bacterial challenge. Mice were sacrificed 24 hours later and the number of bacteria in the peritoneal lavage fluid was counted. (A) Different doses of DP7-C formulation (DP7-C dosage of 0.1 or 2.5 mg/kg) were administered by intravenous injection. (B) Efficacy of the treatment of DP7-C formulations in combination with AZT. AZT-LPs (AZT dosage of 1.25 mg/kg), D-LPs (DP7-C dosage of 2.5 mg/kg), and AZT-D-LPs (dosage of 1.25 mg AZT/kg and 2.5 mg DP7-C/kg) were administered by intravenous injection. *P<0.05 and ***P<0.001 vs the untreated group, respectively.
Abbreviations: AZT, azithromycin; AZT-D-LPs, DP7-C-modified AZT-loaded LPs; AZT-LPs, AZT-modified liposomes; CFU, colony-forming unit; D-LPs, DP7-C-modified blank LPs; S. aureus, Staphylococcus aureus.
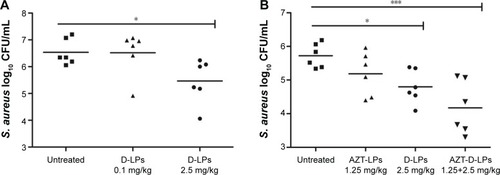
Figure 4 The mechanism of DP7-C formulations.
Notes: (A) Gene expression of cytokines/chemokines in human PBMCs after DP7-C formulations administration. Human PBMCs were incubated with or without DP7-C formulations (DP7-C dosage of 120 μg/mL) for 6 hours, and RNA from subsequently isolated PBMCs was used for qPCR analysis. (B and C) DP7-C formulations modulate the LPS-induced cytokines/chemokines effects in human (B) and mouse (C) PBMCs. The cells were pretreated with or without DP7-C formulations for 60 minutes before stimulated with LPS (10 ng/mL) for 5 hours, and the gene expression of LPS-induced cytokines/chemokines were measured by qPCR. n=3, mean ± standard error; *P<0.05, **P<0.01, and ***P<0.001 vs the untreated group, respectively.
Abbreviations: G-CSF, granulocyte colony-stimulating factor; IFN-γ, interferon gamma; IL, interleukin; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein-1; PBMCs, peripheral blood mononuclear cells; qPCR, quantitative real-time polymerase chain reaction; TNF-α, tumor necrosis factor-alpha.
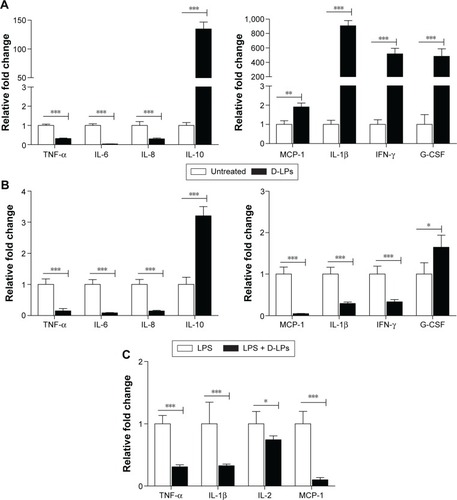
Figure 5 Histological analysis of the main organs of BALB/c mice after treatment with AZT and/or DP7-C liposomes (DP7-C dosage of 2.5 mg/kg and AZT dosage of 1.25 mg/kg).
Abbreviations: AZT, azithromycin; AZT-D-LPs, DP7-C-modified AZT-loaded LPs; AZT-LPs, AZT-modified liposomes; D-LPs, DP7-C-modified blank LPs.
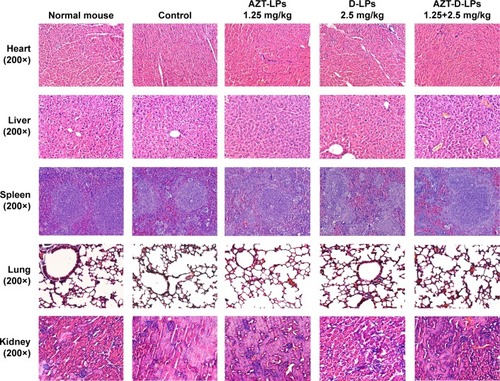
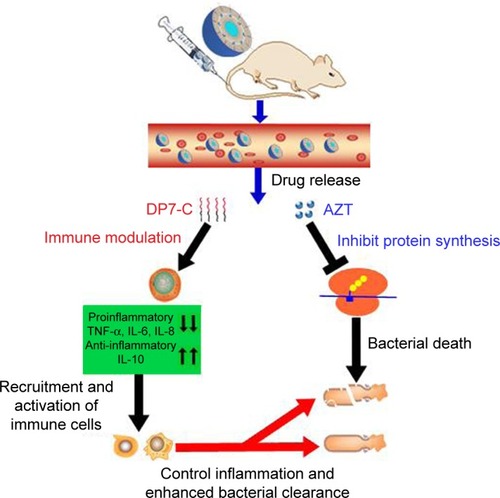
Figure 6 Proposed mechanism of action of AZT-D-LPs.
Notes: Under infected conditions, after IV injection, both DP7-C and AZT were released from AZT-D-LPs formulations. The DP7-C selectively modulates innate immune response, and this results in a supressed inflammatory response that includes the downregulation of proinflammatory cytokines, such as TNF-α, IL-6, and IL-8, and a variety of chemokines. Meanwhile, the anti-inflammatory cytokine IL-10 was upregulated to neutralize the harmful inflammation, and effector cells and cytokines are recruited to the infection sites and controlled the infections. In addition, the released azithromycin binds the 50S ribosomal subunit of bacteria and inhibits the bacterial protein synthesis,Citation32–Citation35 resulting in bacterial death, and the DP7-C enhanced innate immune response collaborated with AZT more effectively clear bacterial debris.
Abbreviations: AZT, azithromycin; AZT-D-LPs, DP7-C-modified AZT-loaded liposomes; IL, interleukin; IV, intravenous; TNF-α, tumor necrosis factor-alpha.