Figures & data
Figure 3 Solubility of grafted SWCNT–betahistine A) in dimethylformamide (DMF) and dimethylsulfoxide (DMSO) and B) in H2O.

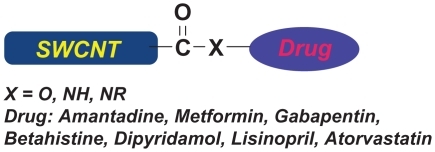
Table 1 Covalent grafting of various drugs to SWCNT
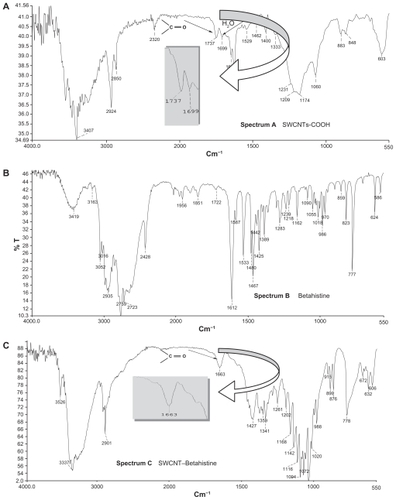
Table 2 Infrared absorption bands obtained from the acid-treated SWCNTs and drugs grafted to SWCNTs’ solid samples
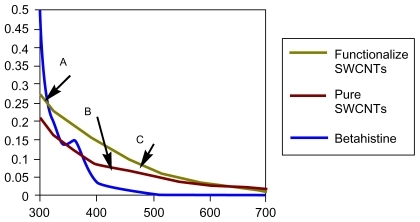
Figure 5 Ultraviolet-visible absorption spectra of A) betahistine in dimethylformamide (DMF), B) pure SWCNTs and, C) grafted SWCNT–betahistine (functionalized SWCNTs).