Figures & data
Figure 1 Schematic representation of the FA-conjugated cisplatin(IV) prodrug MSN platform developed in this work.
Notes: The MSN-delivery system contains cisplatin(IV) prodrug chemically attached to the surface of the nanoparticle and is decorated with FA-PEG-NH2 that targets FA receptors overexpressed in HeLa cancer cells. The FA-conjugated-cisplatin(IV) prodrug MSNs are internalized through a receptor-mediated endocytosis mechanism, escape from lysosomes, and release the cisplatin, due to the highly reducing environment found in the cytosol of cancer cells. The released cisplatin travels to the cell nucleus to form DNA adducts, which triggers apoptosis.
Abbreviations: FA, folic acid; MSN, mesoporous silica nanoparticle; PEG, polyethylene glycol.
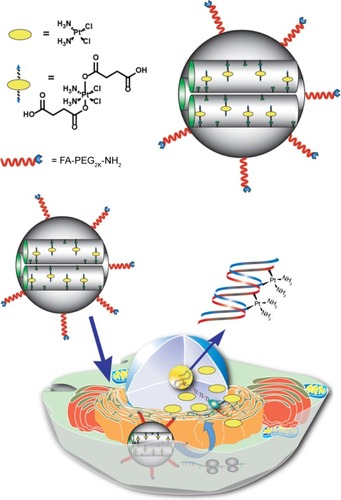
Table 1 Structural properties of MSN materials synthesized in this work
Figure 2 Structural properties of MSN materials.
Notes: (A) Nitrogen sorption isotherm of AP-MSNs; (B) nitrogen sorption isotherm of cisplatin(IV)-MSNs; (C) volume-weighted hydrodynamic diameter distributions of MSN material; (D) SEM image of AP-MSNs.
Abbreviations: AP, aminopropyl; MSNs, mesoporous silica nanoparticles; SEM, scanning electron microscopy; FA, folic acid; MeO, methoxy.
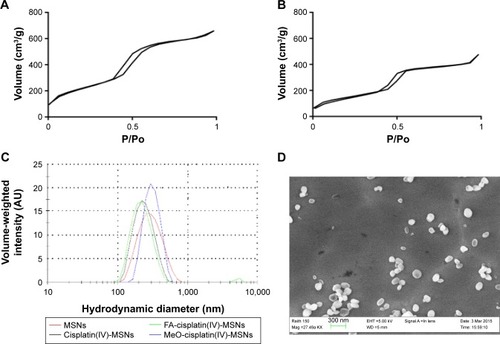
Figure 3 ESI mass spectra for the reduction of FA-cisplatin(IV)-MSNs to cisplatin.
Notes: FA-cisplatin(IV)-MSNs were incubated in the presence (A) or absence (B) of ascorbic acid in an aqueous solution containing guanosine for 24 hours at 37°C. Then, the formation of the cis-Pt[(NH3)2Cl(guanosine)]+ cation (m/z =547.1) was determined by ESI mass spectrometry. Abbreviations: ESI, electrospray ionization; FA, folic acid; MSNs, mesoporous silica nanoparticles.
![Figure 3 ESI mass spectra for the reduction of FA-cisplatin(IV)-MSNs to cisplatin.Notes: FA-cisplatin(IV)-MSNs were incubated in the presence (A) or absence (B) of ascorbic acid in an aqueous solution containing guanosine for 24 hours at 37°C. Then, the formation of the cis-Pt[(NH3)2Cl(guanosine)]+ cation (m/z =547.1) was determined by ESI mass spectrometry. Abbreviations: ESI, electrospray ionization; FA, folic acid; MSNs, mesoporous silica nanoparticles.](/cms/asset/0346ab3d-f4fe-41b5-abff-a91cdc10f04c/dijn_a_118196_f0003_b.jpg)
Figure 4 Internalization results by flow cytometry.
Notes: HeLa cancer cells were exposed to 100 μg/mL of FA-FITC-MSNs or MeO-FITC-MSNs for 6 hours. Then, normalized mean fluorescence was obtained. Error bars represent the standard deviation of three independent experiments.
Abbreviations: FA, folic acid; FITC, fluorescein isothiocyanate; MSNs, mesoporous silica nanoparticles; MeO, methoxy.
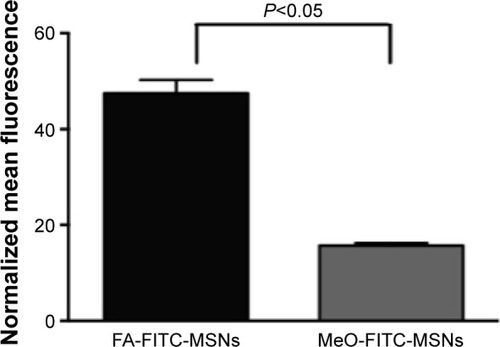
Figure 5 Localization of FA-FITC-MSNs or MeO-FITC-MSNs in HeLa cancer cells.
Notes: Cells were exposed to 100 μg/mL of MSN materials for 6 hours. (A, E) Nucleus (blue); (B, F) FITC-MSNs (green); (C, G) DIC; (D, H) merged. Scale bars represent 5 μm.
Abbreviations: FA, folic acid; FITC, fluorescein isothiocyanate; MSNs, mesoporous silica nanoparticles; MeO, methoxy; DIC, differential interference contrast.
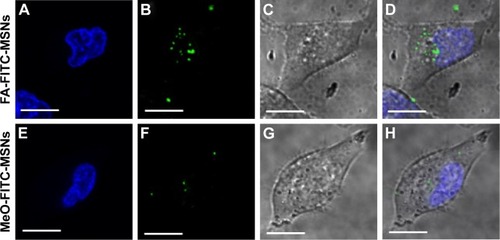
Figure 6 Escape of FA-FITC-MSNs from endolysosome compartments.
Notes: HeLa cells were treated with 100 μg/mL of FA-FITC-MSNs for 30 minutes, 1 hour, or 3 hours. (A, E, I) Nanoparticles (green); (B, F, J) endolysosome compartments (red); (C, G, K) DIC; (D, H, L) merged, overlapping of red and green (yellow). Scale bars represent 5 μm.
Abbreviations: FA, folic acid; FITC, fluorescein isothiocyanate; MSNs, mesoporous silica nanoparticles; DIC, differential interference contrast.
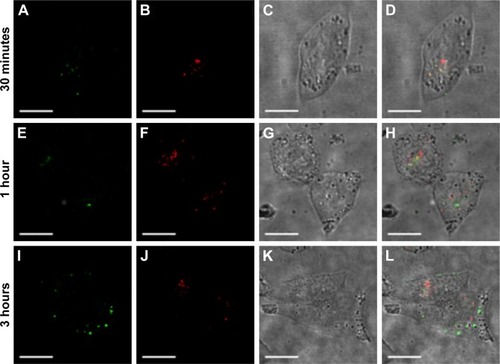
Figure 7 Cisplatin-adduct measurement by flow cytometry.
Notes: HeLa cells were exposed to 6.5 μM of platinum for 24 hours, fixed in ethanol, incubated with an anticisplatin-modified DNA antibody and labeled with an antirat FITC-labeled antibody. Error bars represent the standard deviation of five independent experiments.
Abbreviations: FITC, fluorescein isothiocyanate; MeO, methoxy; MSNs, mesoporous silica nanoparticles; FA, folic acid.
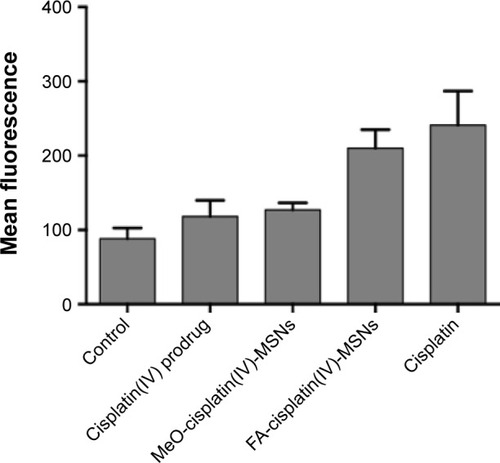
Figure 8 Dose–response curve.
Notes: HeLa cells were exposed to varying concentrations of platinum for 48 hours. Error bars represent the standard deviation of five independent experiments.
Abbreviations: FA, folic acid; MSNs, mesoporous silica nanoparticles; MeO, methoxy.
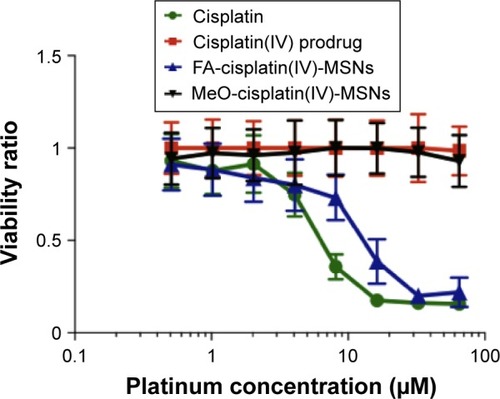
Figure S1 Schematic representation of the synthesis of FA-PEG-NH2.
Notes: To convert the alcohol group in the PEG2K chain to amine group, the polymer was reacted with MsCl in anhydrous THF to afford a good leaving group. The PEG mesylate derivative was reacted with NaN3, a strong nucleophile, to afford the azido-PEG product. To convert the diazido-PEG2K derivative to the corresponding diamino-PEG2K, a mild reduction was carried out by following Staudinger reaction conditions. The diazido-PEG2K chain was reacted with PPh3 in anhydrous THF to generate the corresponding iminophosphorane. An aqueous workup led to the desired diamino-PEG2K derivative and the very stable phosphine oxide as by-product. The diamino-PEG polymer was further reacted with FA to afford the FA-PEG-NH2 targeting ligand. Firstly, the carboxylic acid group in the FA molecule was activated by using a coupling reaction in the presence of EDC and NHS. The succinimide ester derivative enhanced the reactivity of the FA toward nucleophilic substitution by amines.
Abbreviations: FA, folic acid; PEG, polyethylene glycol; THF, tetrahydrofuran; EDC, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride; NHS, N-hydroxysuccinimide; DIPEA, diisopropylethylamine; DMAP, dimethylaminopyridine; DMSO, dimethyl sulfoxide.
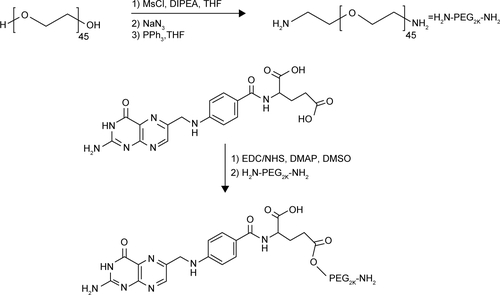
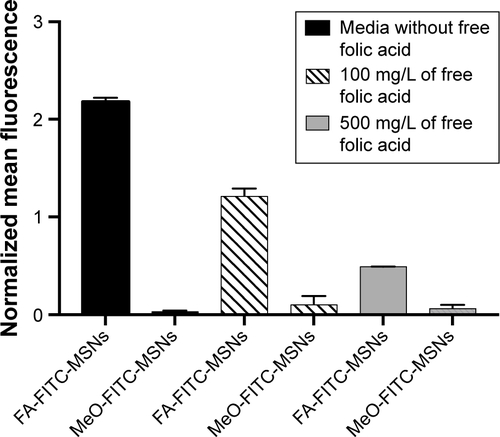
Figure S2 Internalization results by flow cytometry.
Notes: HeLa cancer cells were incubated with media containing 100 mg/L or 500 mg/L of free FA for 24 hours and exposed to 50 μg/mL of FA-FITC-MSNs or MeO-FITC-MSNs for 4 hours. Then, normalized mean fluorescence was obtained. Error bars represent the standard deviation of three independent experiments.
Abbreviations: FA, folic acid; FITC, fluorescein isothiocyanate; MSNs, mesoporous silica nanoparticles; MeO, methoxy.