Figures & data
Figure 1 FTIR spectra of (A) PCL20-ss-PMPC5, (B) PCL20-ss-PMPC10 and (C) PCL40-ss-PMPC10.
Abbreviations: FTIR, Fourier transform infrared; PCL-ss-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide.
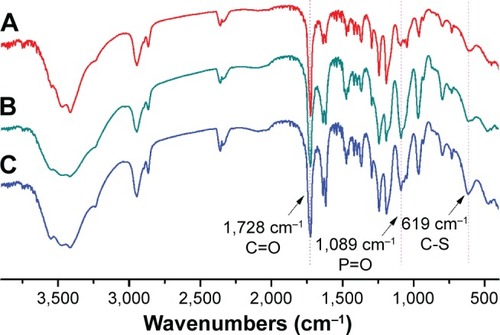
Table 1 Synthesis and characteristics of polymers
Figure 2 1H NMR spectra of (A) PCL20-ss-PMPC5, (B) PCL20-ss-PMPC10 and (C) PCL40-ss-PMPC10 in CDCl3 and CD3OD (v/v =2:1).
Abbreviations: NMR, nuclear magnetic resonance; PCL-ss-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide.
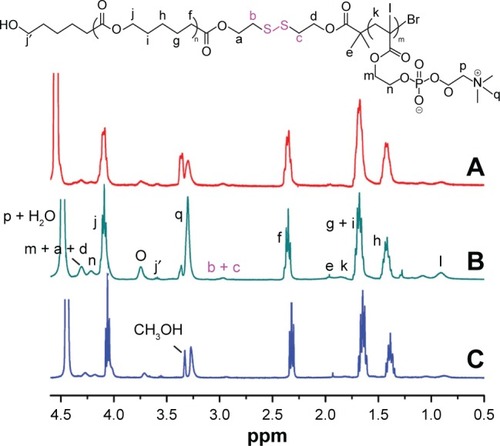
Figure 3 DSC curve of different polymers for heating (A) and cooling (B).
Abbreviations: PCL-ss-iBuBr, poly(ε-caprolactone) with OH-ss-iBuBr opening-ring polymerization; DSC, differential scanning calorimetry; PCL-ss-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide; PCL-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) without disulfide.
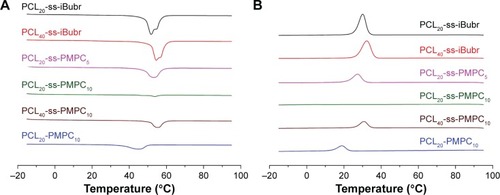
Table 2 Preparation and characteristics of blank micelles and DOX-loaded micelles
Figure 4 Measurement of CMC for different block polymers.
Abbreviations: CMC, critical micelle concentration; PCL-ss-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide.
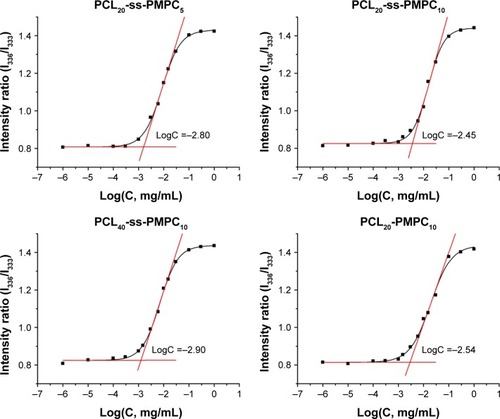
Figure 5 Size distribution of blank micelles (A) and DOX-loaded micelles (B). Diameter change of PCL20-ss-PMPC10 (C) and PCL20-PMPC10 (D) micelles in response to 10 mM DTT under 37°C measured by DLS.
Abbreviations: DOX, doxorubicin; DTT, DL-dithiothreitol; DLS, dynamic light scattering; PCL-ss-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide; PCL-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) without disulfide.
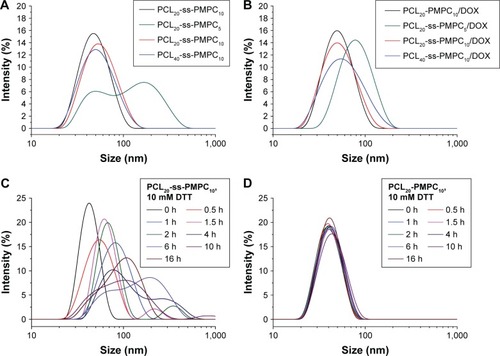
Figure 6 In vitro drug release of PCL-ss-PMPC/DOX micelles (A–C) and PCL-PMPC micelles (D) in PBS (pH =7.4, 0.01 M) with 10 mM DTT or without DTT at 37°C.
Abbreviations: DOX, doxorubicin; PBS, phosphate-buffered saline; DTT, DL-dithiothreitol; PCL-ss-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide; PCL-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) without disulfide.
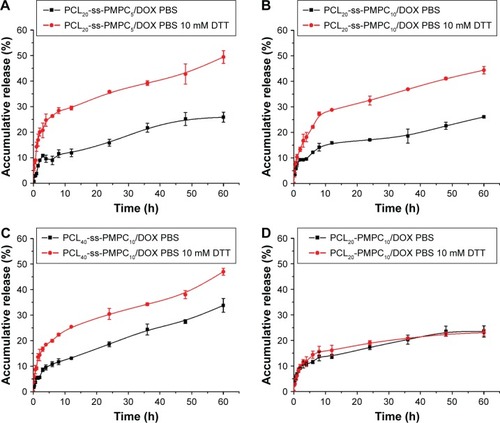
Figure 7 In vitro cytotoxicity of blank and DOX-loaded micelles.
Notes: Cell viability of HeLa cells (A) and L929 cells (B) incubated with different blank micelles for 72 h at 37°C in 5% CO2 atmosphere. Cell viability of HeLa cells and L929 cells incubated with different DOX-loaded micelles for 48 and 96 h (n=5, SD <0.06; *P<0.05, **P<0.01, ***P<0.001) (C). In vitro cytotoxicity of different DOX-loaded micelles with various DOX concentrations incubated with HeLa cells (D).
Abbreviations: DOX, doxorubicin; IC50, half inhibitory concentration; PCL-ss-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide; PCL-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) without disulfide.
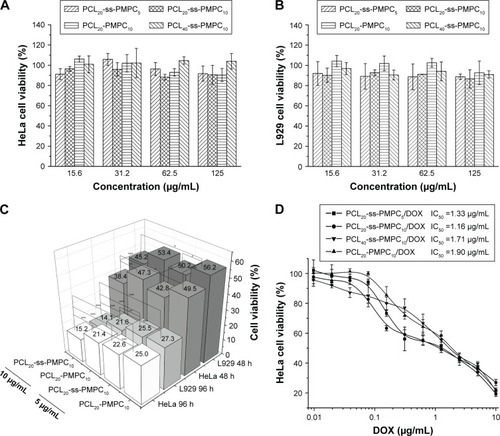
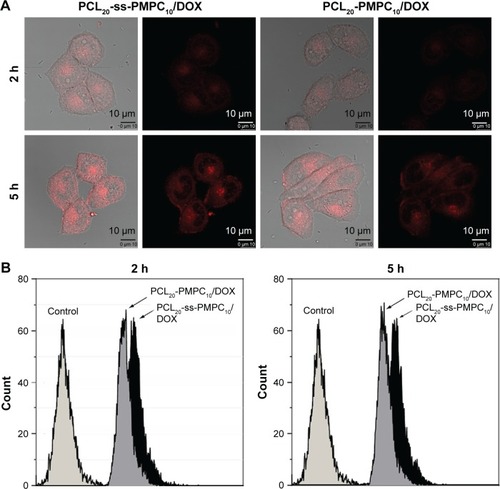
Figure 8 Cellular uptake and intracellular drug release by CLSM (A) and FCM (B).
Note: HeLa cells were incubated with DOX-loaded micelles with the final DOX concentration of 10 μg/mL at 37°C for 2 or 5 h.
Abbreviations: CLSM, confocal laser scanning microscopy; FCM, flow cytometry; DOX, doxorubicin; PCL-ss-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide; PCL-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) without disulfide.
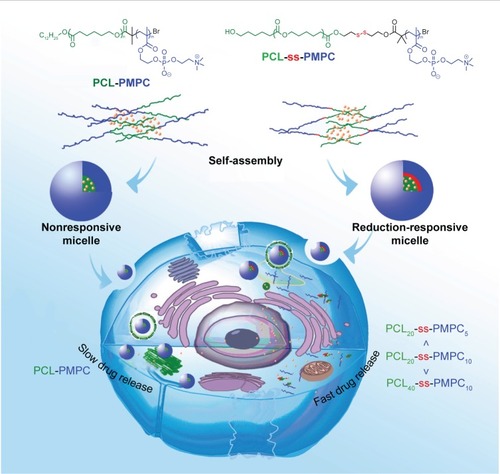
Scheme 1 Illustration for intracellular drug delivery of reduction-responsive micelles with bioinspired phosphorylcholine as hydrophilic chains.
Abbreviations: PCL-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) without disulfide; PCL-ss-PMPC, poly(ε-caprolactone)-b-poly(2-methacryloyloxyethyl phosphorylcholine) with disulfide.