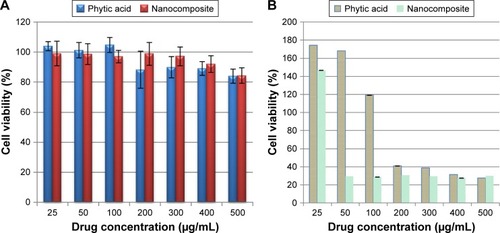
Figures & data
Figure 1 Powder X-ray diffraction patterns of iron oxide magnetic nanoparticles (A), PTA-CS-MNP nanocomposite (B), and phytic acid sodium salt (C).
Abbreviation: PTA-CS-MNP, phytic acid-chitosan-iron oxide nanocomposite.
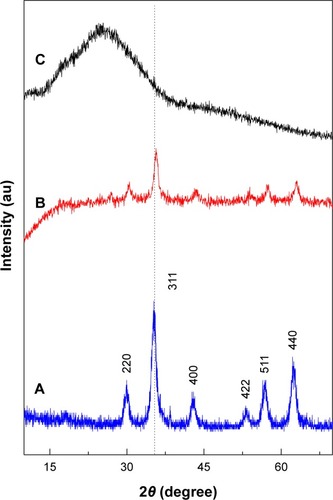
Figure 2 Fourier transform infrared spectra of iron oxide magnetic nanoparticles (A), PTA-CS-MNP nanocomposite (B), and phytic acid sodium salt (C).
Abbreviation: PTA-CS-MNP, phytic acid-chitosan-iron oxide nanocomposite.
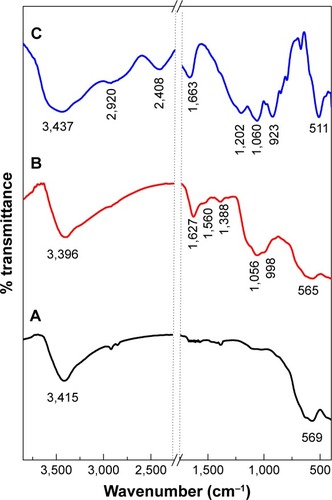
Figure 3 Schematic representation of the interaction between iron oxide magnetic nanoparticles, chitosan (A), and phytic acid (B) in the PTA-CS-MNP nanocomposite.
Abbreviation: PTA-CS-MNP, phytic acid-chitosan-iron oxide nanocomposite.
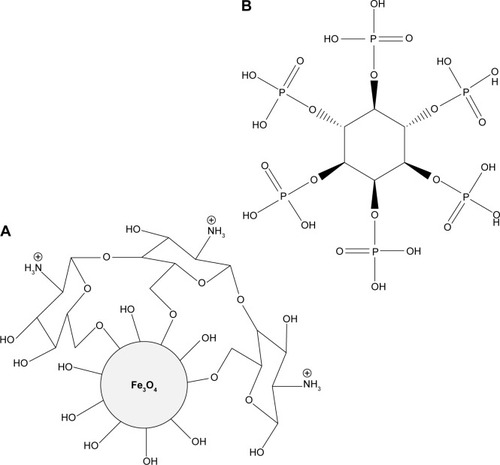
Figure 4 TGA/DTG thermograms of phytic acid sodium salt (A), iron oxide magnetic nanoparticles (B), and PTA-CS-MNP nanocomposite (C).
Abbreviations: PTA-CS-MNP, phytic acid-chitosan-iron oxide nanocomposite; TGA/DTG, thermogravimetric and differential thermogravimetric analyses.
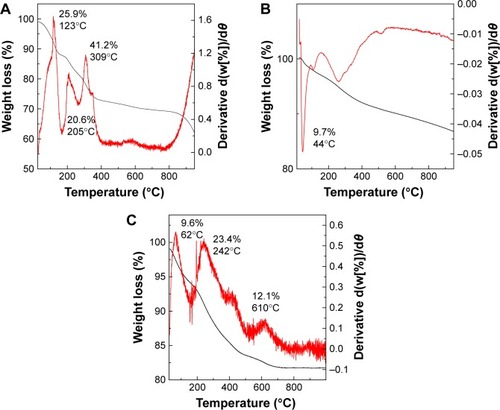
Figure 5 Magnetization curves of iron oxide magnetic nanoparticles (A) and phytic acid-loaded chitosan-iron oxide magnetic nanoparticles (B) recorded at room temperature.
Abbreviation: Oe, oersted.
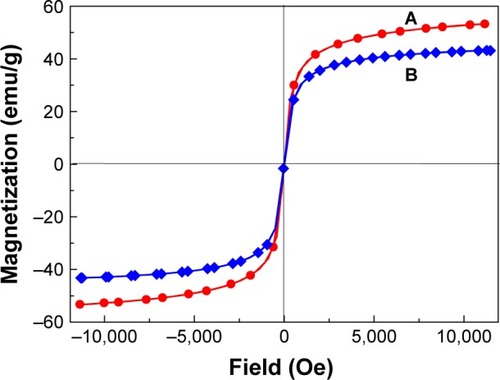
Figure 6 TEM micrographs of iron oxide magnetic nanoparticles (A), chitosan-iron oxide magnetic nanoparticles (B), and PTA-CS-MNP nanocomposite (C). Particle size distribution of iron oxide magnetic nanoparticles (D), chitosan-iron oxide magnetic nanoparticles (E), and PTA-CS-MNP nanocomposite (F).
Abbreviations: PTA-CS-MNP, phytic acid-chitosan-iron oxide nanocomposite; TEM, transmission electron microscopy.
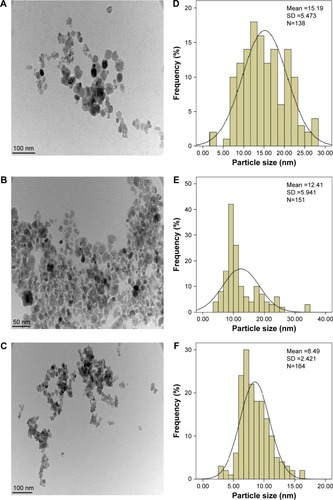
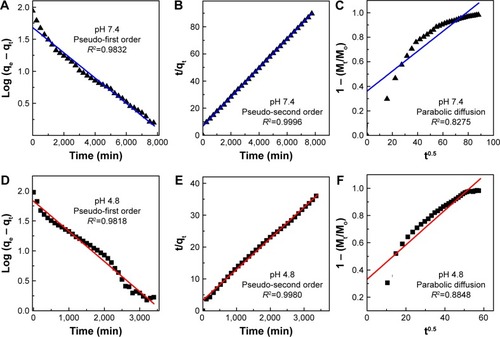
Figure 7 Release profiles of phytic acid from PTA-CS-MNP nanocomposite into phosphate-buffered saline solution at pH 7.4 and pH 4.8. The inset shows the release profiles of a physical mixture of phytic acid sodium salt with chitosan and iron oxide nanoparticles into phosphate-buffered saline solution at pH 7.4 and pH 4.8.
Abbreviation: PTA-CS-MNP, phytic acid-chitosan-iron oxide nanocomposite.
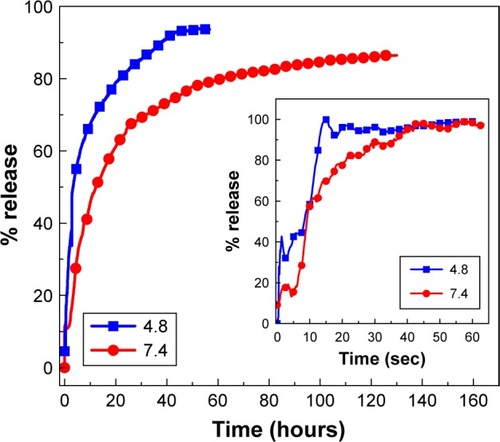
Table 1 Correlation coefficient (R2), rate constant (k), and half-life (t1/2) values obtained by fitting the release data of phytic acid from PTA-CS-MNP nanocomposite into phosphate-buffered saline solution at pH 4.8 and 7.4