Figures & data
Figure 1 Construction and gel analysis of DNA-binding carrier protein Her-NLS. A) Schematic representation of chimeric fusion construct encoding the Her-NLS gene. The motifs harboring the NLS sequence (DPKKKRKV)3 were cloned in-frame with heregulin and expressed as N-terminal His-tag fusion protein in Escherichia coli. The plasmid is designated as pHer-NLS (not drawn to scale). B) Primary amino acid sequence (single amino acid code is color-coded) of the fusion protein Her-NLS based on DNA sequencing. NLS (blue), heregulin (red), and enterokinase cleavage site (green). The N-terminal His-tag is also depicted (cyan). The predicted molecular weight based on the sequence given is 34,699 Daltons. C) Sodium dodecyl sulfate-polyacrylamide gel electrophoretic analysis of the purified fusion proteins. Lanes correspond to Her-NLS after desalting from PD-10 columns. The molecular weight of the expressed proteins is 34,699 Daltons. Lane on the extreme right shows the immunoblot analysis of Her-NLS after electrophoresis and transfer. The 6 × His tagged proteins were detected with monoclonal anti-His antibody followed by secondary antibody goat antimouse IgG alkaline phosphatase conjugate. Detection was by nitro blue tetrazolium chloride and 5-bromo-4-chloro-3-indoyl-1-phosphate treatment.
Abbreviation: NLS, nuclear localization sequence.
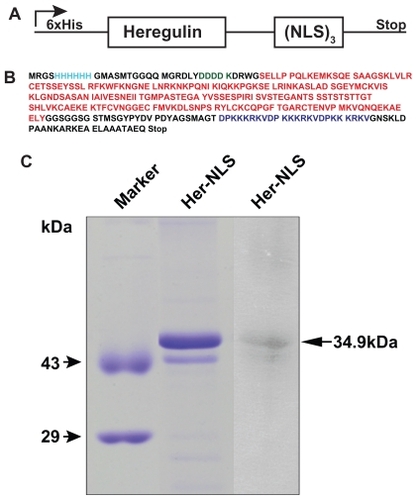
Figure 2 DNase I protection assay of the fusion protein Her-NLS. Plasmid pCMV- βgal (0.2μg) was incubated in 1 × Hepes buffer saline (pH 7.4) with increasing amounts of the purified protein corresponding to the charge ratio indicated above lanes 3, 5, and 7 (minus DNase I) and 4, 6, and 8 (plus DNase I). The samples were loaded along with control plasmid DNA (lane 1) and in the presence of DNase I (lane 2). Lanes 9 and 11 indicate untreated DNA:protamine sulfate complexes, while lanes 10 and 12 correspond to DNA:protamine sulfate complexes treated with DNase I. DNase I-treated samples were extracted with phenol-chloroform and loaded along with untreated samples and electrophoresed on 1% agarose gel in Tris-acetate-ethylenediaminetetraacetate buffer. Gels were visualized by staining with ethidium bromide.
Abbreviation: NLS, nuclear localization sequence.
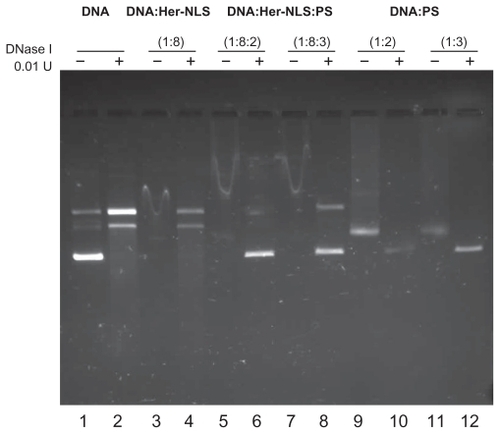
Figure 3 Confocal microscopy of uptake of transfection-competent complexes into MDA-MB-453 versus MDA-MB-231 cells. Confocal microscope analysis of uptake of Oregon green labeled-DNA:Her-NLS fusion protein complexes into cells. Peptide-mediated uptake of labeled DNA into MDA-MB-453 and MDA-MB-231 is seen as cell-associated fluorescence. DNA 0.9 μg was used to make the transfection complexes at a charge ratio of 1:8:2 (DNA:peptide:protamine sulfate) and applied to cells in the absence of serum. Uptake of the complexes is for three hours’ duration, after which the cells were counterstained with Hoechst 33258, and visualized by confocal microscopy as described.
Abbreviation: NLS, nuclear localization sequence.
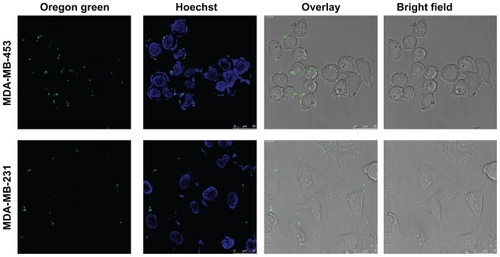
Figure 4 Expression of HER2/3 receptors in MDA-MB-231 and MDA-MB-453 cells by Western blotting. MDA-MB-453 and MDA-MB-231 cell lysates, normalized for protein, were analyzed on 6% sodium dodecyl sulfate-polyacrylamide gel electrophoretic analysis and blotted onto a nitrocellulose membrane. The blot was probed using primary antibody ErbB2 (intracellular domain) against HER2/3 receptors and treated with the secondary antibody antimouse IgG conjugated to alkaline phosphatase. Chinese hamster ovary cells were loaded as additional controls. Lysates for probing with antibodies to glyceraldehyde-3-phosphate dehydrogenase was also normalized and loaded separately on a 12% gel, and served as an internal loading control.
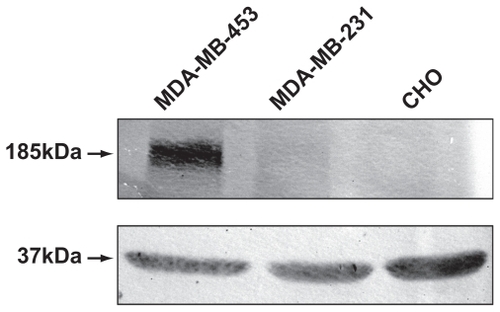
Figure 5 Transient transfection of MDA-MB-453 cells versus MDA-MB-231 cells with Her-NLS fusion protein. Complexes were prepared with the fusion protein at a charge ratio of 1:8 and 1:8:2 of DNA+Her-NLS and DNA+Her-NLS+PS respectively. β-galactosidase activity was determined 48 hours post transfection. The plot depicts normalized mU β-galactosidase activity from plasmid pCMV β-gal.
Abbreviations: NLS, nuclear localization sequence; PS, protamine sulphate.
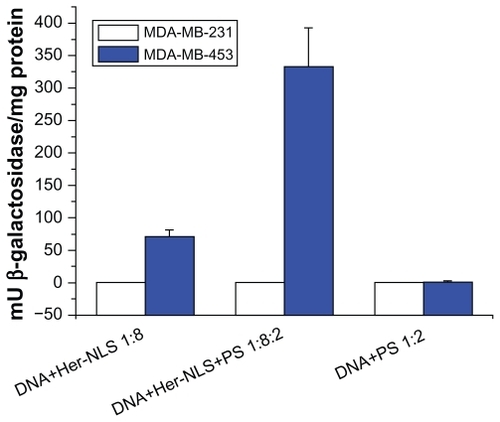
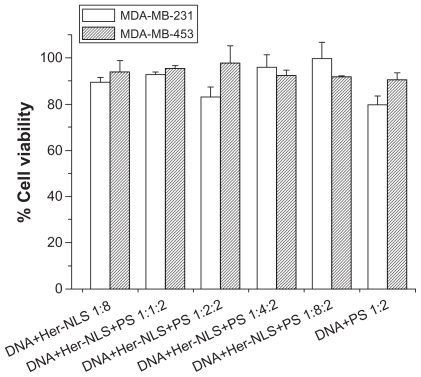
Figure 6 Toxicity assay. The cytotoxicity of DNA-Her-NLS:protamine sulfate complexes at varying concentrations of Her-NLS was evaluated in MDA-MB-231 and MDA-MB-453 cells. The concentration of DNA:protamine sulfate was kept constant at 1:2 charge ratios. Toxicity was measured as percent viability from absorption values obtained using reduced formazan, with cells in the absence of the complexes taken to be 100.
Abbreviations: NLS, nuclear localization sequence; PS, protamine sulphate.