Figures & data
Figure 1 Comparison of gel permeation chromatograms of (A) PCL-Br, (B) PCL-b-P(OEGMA-co-AzPMA) with tetrahydrofuran used as the elute and (C) shell cross-linked micelles with dimethylformamide used as the elute.
Abbreviations: AzPMA, 3-azidopropyl methacrylate; M, molecular weight; Mn, average number molecular weight; OEGMA, oligo(ethylene glycol) ethyl methacrylate; PCL, polycaprolactone; PDI, polydispersity index.
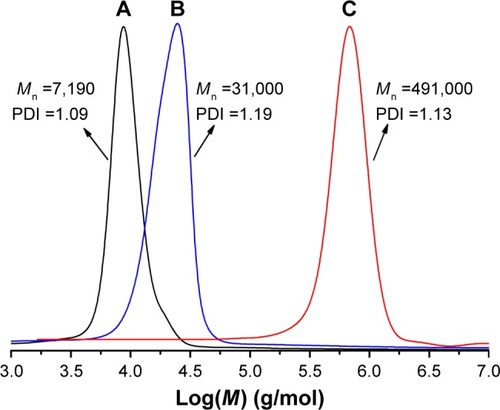
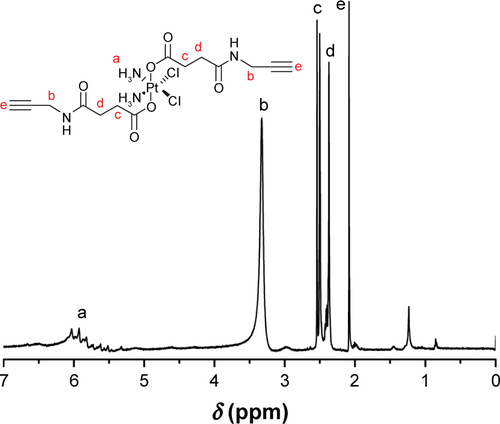
Figure 2 Proton nuclear magnetic resonance spectra of (A) PCL-Br and (B) PCL-b-P(OEGMA-co-AzPMA) in CDCl3.
Abbreviations: AzPMA, 3-azidopropyl methacrylate; OEGMA, oligo(ethylene glycol) ethyl methacrylate; PCL, polycaprolactone.
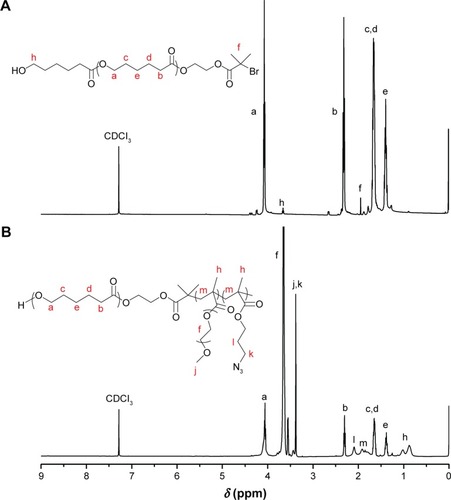
Figure 3 Fourier transform infrared spectra of (A) PCL-Br, (B) PCL-b-P(OEGMA-co-AzPMA) and (C) shell cross-linked micelles.
Abbreviations: AzPMA, 3-azidopropyl methacrylate; OEGMA, oligo(ethylene glycol) ethyl methacrylate; PCL, polycaprolactone.
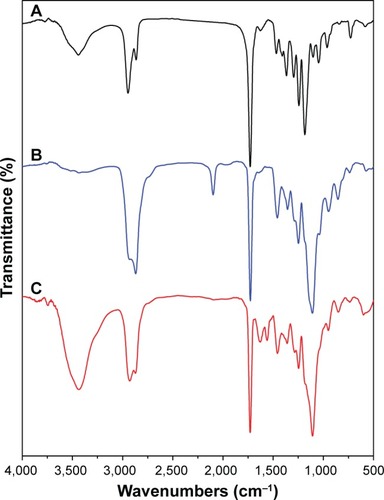
Figure 4 Transmission electron microscopy image of (A) NCL micelles and (B) SCL micelles. Dynamic light scattering curves of NCL and SCL micelles in water (C) and in DMF (D).
Abbreviations: DMF, dimethylformamide; NCL, non-cross-linked; SCL, shell cross-linked.
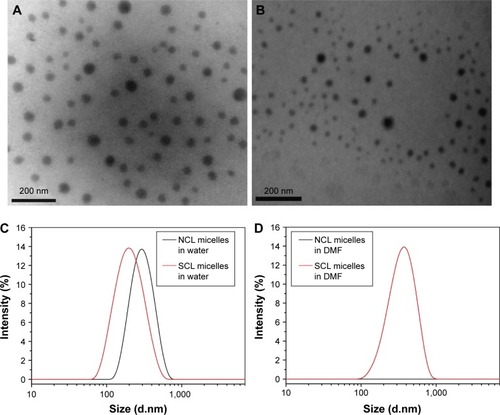
Figure 5 Thermogravimetric analysis curve of cisplatin, PCL-b-P(OEGMA-co-AzPMA) copolymer and Pt(IV) prodrug-loaded PCL-b-P(OEGMA-co-AzPMA) copolymer.
Abbreviations: AzPMA, 3-azidopropyl methacrylate; OEGMA, oligo(ethylene glycol) ethyl methacrylate; PCL, polycaprolactone; Pt, platinum.
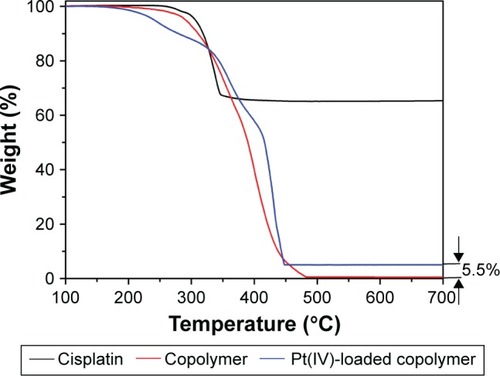
Figure 6 In vitro (A) Pt and (B) DOX release profiles of Pt(IV)- and DOX-loaded shell cross-linked micelles in aqueous solution at (■) pH 7.4, (●) pH 5.5, (▲) pH 7.4 with 5 mM sodium ascorbate and (▼) pH 5.5 with 5 mM sodium ascorbate.
Abbreviations: DOX, doxorubicin; Pt, platinum.
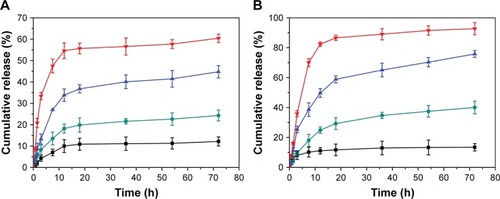
Figure 7 Fluorescence microscopy images of HeLa cells after incubation with DOX- and Pt(IV)-loaded shell cross-linked micelles for 6 h: (A) DOX (red), (B) cell nuclei stained by 4′,6-diamidino-2-phenylindole (blue), (C) bright field and (D) overlay of (A–C).
Abbreviations: DOX, doxorubicin; Pt, platinum.
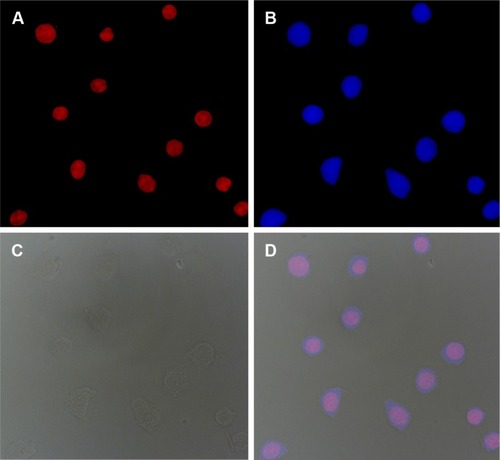
Figure 8 Cell cytotoxicity of PCL-b-P(OEGMA-co-AzPMA) against A357, HeLa and HEK-293 cells with different incubation times of (A) 48 and (B) 72 h.
Abbreviations: AzPMA, 3-azidopropyl methacrylate; OEGMA, oligo(ethylene glycol) ethyl methacrylate; PCL, polycaprolactone.
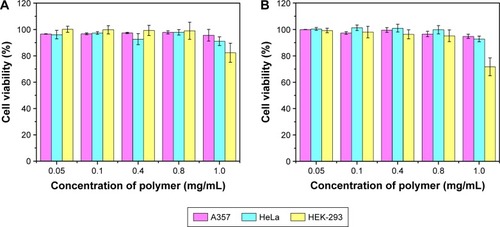
Table 1 IC50 values of single drugs and shell cross-linked micelles loaded with DOX and Pt(IV) corresponding to A357 and HeLa cells at different incubation times
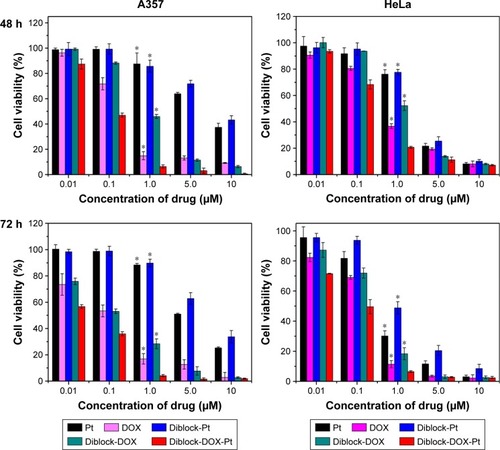
Figure 9 In vitro cell viability of A357 and HeLa cells against single drugs and shell cross-linked micelles loaded with DOX and Pt(IV) at different concentrations and incubation times. *P<0.05 compared with diblock-DOX-Pt.
Abbreviations: DOX, doxorubicin; Pt, platinum.
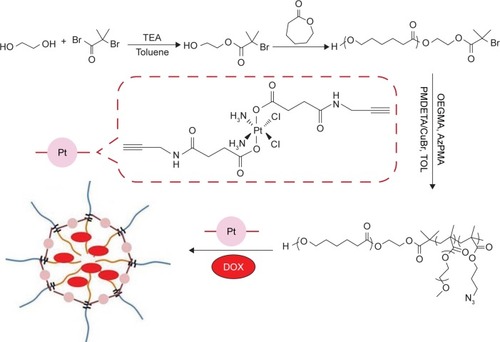
Scheme 1 Synthesis routes employed for the preparation of dual-drug-loaded shell cross-linked micelles.
Abbreviations: AzPMA, 3-azidopropyl methacrylate; DOX, doxorubicin; OEGMA, oligo(ethylene glycol) ethyl methacrylate; PMDETA, N,N,N′,N″,N′″-pentamethyldiethylenetriamine; Pt, platinum; TEA, triethyl alcohol; TOL, toluene.