Figures & data
Table 1 Equilibrium solubility of ravuconazole in different SEDDS excipients
Table 2 Effects of the variations in oil, surfactant, cosurfactant, and cosolvent proportions on the physicochemical properties of blank SEDDS
Table 3 Effects of RAV incorporation on the physicochemical properties of SEDDS with 70% Miglyol
Figure 2 Asymmetric flow field-flow fractionation data.
Notes: Fractionation data of blank SEDDS and RAV–SEDDS F5 formulations at different dilutions (1:200, 1:400, and 1:800) at 37°C with size characterization by multiangle laser light scattering (Dg) and dynamic light scattering (Dh) detectors coupled in series. The black arrow indicates the main peak of the ravuconazole–SEDDS, (tR =16.3 min, which corresponds to Dh of ~165 nm).
Abbreviations: au, arbitrary unit; RAV, ravuconazole; SEDDS, self-emulsifying drug delivery system; UV, ultraviolet; tR, retention time.
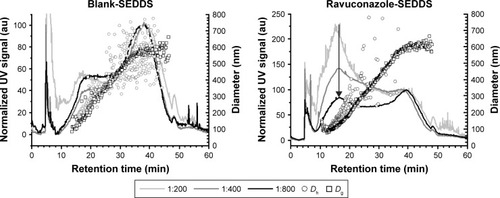
Table 4 RAV–SEDDS robustness to dilution in water at 37°C (10 mg/mL) and influence of pH of different media
Figure 3 Relationship between Labrasol (surfactant) concentration and ravuconazole solubility at 37°C in PBS (pH 6.8).
Notes: S0 is the intrinsic solubility in PBS (<1 µg/mL). The dotted line is the linear regression (r2=0.9818, S =66.6× % Labrasol − 8.368). Data are expressed as mean ± standard deviation (n=3).
Abbreviation: PBS, phosphate-buffered saline.
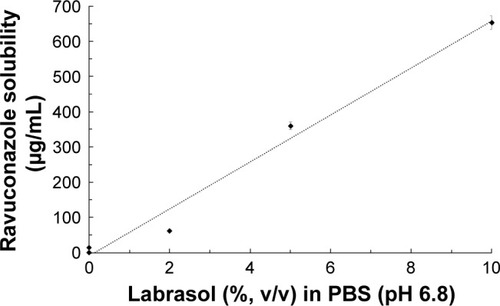
Figure 4 Mean release/dissolution profile versus time of RAV in aqueous buffer at 37°C in vitro, as SEDDS formulation (F5, mean diameter of nanoemulsion: 187 nm) or as free powder.
Notes: External dissolution medium I is PBS (pH 6.8) and medium II is PBS (pH 6.8) plus 5% (v/v) of surfactant (sink condition). Mean ± standard deviation data for the percentage cumulative amount of RAV released in vitro. The insert graph represent the first timepoints.
Abbreviations: PBS, phosphate-buffered saline; RAV, ravuconazole; SEDDS, self-emulsifying drug delivery system.
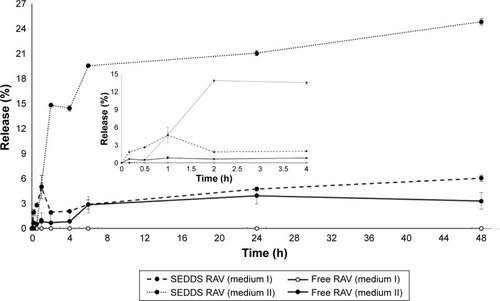
Table 5 Stability of blank SEDDS and RAV–SEDDS (10 mg/mL of RAV) after 6 months at room temperature (25°C)
Table 6 Clinical signs of general toxicity of blank SEDDS in mice after oral administration (gavage) for 20 days
Figure 5 Bodyweight variation from initial time after repeated administrations of different SEDDS formulations for 20 days by the oral route following the classical protocol for T. cruzi-infected mice.
Notes: (A) Healthy mice received blank SEDDS (BL-SEDDS) with varying Labrasol contents. (B) Treatment was given to T. cruzi-infected mice at fixed Labrasol concentration (formulation F5) with RAV-loaded RAV–SEDDS and free RAV (RAV). Horizontal bars refer to significant differences between groups; *P<0.005.
Abbreviations: BL-SEDDS, blank SEDDS; RAV, ravuconazole; SEDDS, self-emulsifying drug delivery system; T. cruzi, Trypanosoma cruzi.
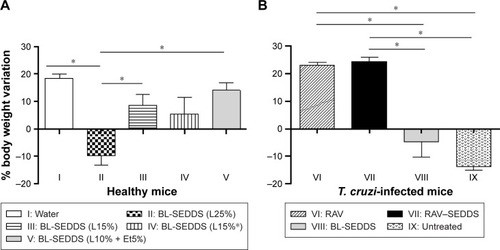
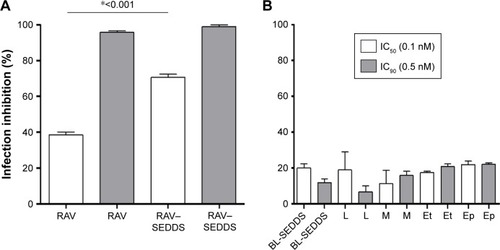
Figure 6 Effects of (A) free RAV, RAV–SEDDS (10 mg/mL) and (B) blank SEDDS (BL-SEDDS) and each SEDDS excipient tested separately on infection inhibition of T. cruzi (Y strain) amastigotes in H9c2 cells.
Notes: M: Miglyol 810N; Ep: Epikuron 170; L: Labrasol; and Et: ethanol were tested at two doses equivalent to ravuconazole IC50 and IC90, (0.1 nM and 0.5 nM, respectively) under our experimental conditions. *Indicates significant differences by Tukey’s multiple-comparisons test (P<0.05). IC50 and IC90, the concentration of drug needed to inhibit 50% or 90%, respectively, of parasite growth.
Abbreviations: RAV, ravuconazole; SEDDS, self-emulsifying drug delivery system; T. cruzi, Trypanosoma cruzi.