Figures & data
Figure 1 Schematic illustration of the synthesis procedure of LDM-PLGA/PPF/VEGF shRNA nanocomposites for codelivery of DOX and VEGF shRNA in EMT-6 tumor models.
Notes: (A) The preparation process of PEI-PEG-FA. (B) The construction of PLGA-based polymeric nanoparticles using a double emulsion solvent evaporation method. (C) The transport process of LDM-PLGA/PPF/VEGF shRNA nanocomposites and inhibition of tumor growth through cellular uptake via endocytosis, endosomal escape, intracellular VEGF shRNA, and DOX release.
Abbreviations: DOX, doxorubicin; PLGA, poly(d,l-lactic-co-glycolic acid); PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid; PPF, PEI-PEG-FA; shRNA, small hairpin RNA; VEGF, vascular endothelial growth factor; siRNA, small interfering RNA; QDs, quantum dots; EDC, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride; NHS, N-hydroxysuccinimide.
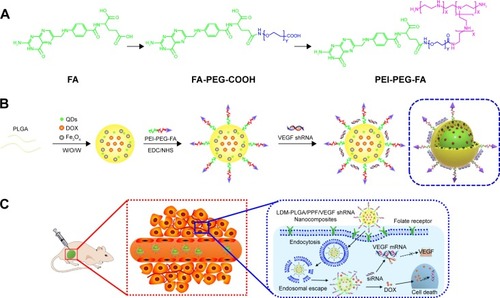
Figure 2 Characterization of the as-synthesized nanoparticles.
Notes: (A) Morphology: (a) Scanning electron microscopy images of the PLGA nanoparticles coloaded with QDs, DOX, and Fe3O4 without modification (LDM-PLGA); (b) PLGA nanoparticles coloaded with QDs, DOX, and Fe3O4 modified with PPF conjugate (LDM-PLGA/PPF); (c) PLGA nanoparticles coloaded with QDs, DOX, and Fe3O4 modified with PPF conjugate and VEGF shRNA (LDM-PLGA/PPF/VEGF shRNA); (d) transmission electron microscopy images of LDM-PLGA. (B) The particle size distributions of (e) LDM-PLGA and (f) LDM-PLGA/PPF, determined by dynamic light scattering. (C) Zeta potentials of LDM-PLGA, LDM-PLGA/PPF, and LDM-PLGA/PPF/VEGF shRNA nanoparticle composites in PBS (pH 7.4) at room temperature (25°C).
Abbreviations: DOX, doxorubicin; PPF, PEI-PEG-FA; PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid; QD, quantum dot; shRNA, small hairpin RNA; VEGF, vascular endothelial growth factor.
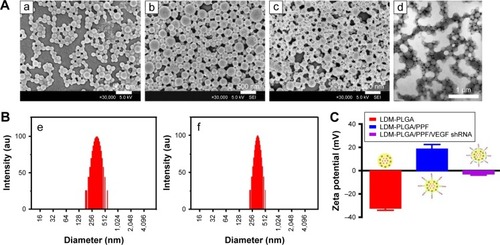
Figure 3 (A) Thermogravimetric analysis of LDM-PLGA and LDM-PLGA/PPF. (B) Agarose gel electrophoresis assay of LDM-PLGA/PPF/VEGF shRNA at various weight ratios of LDM-PLGA/PPF to VEGF shRNA.
Abbreviations: PPF, PEI-PEG-FA; PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid; shRNA, small hairpin RNA; VEGF, vascular endothelial growth factor.
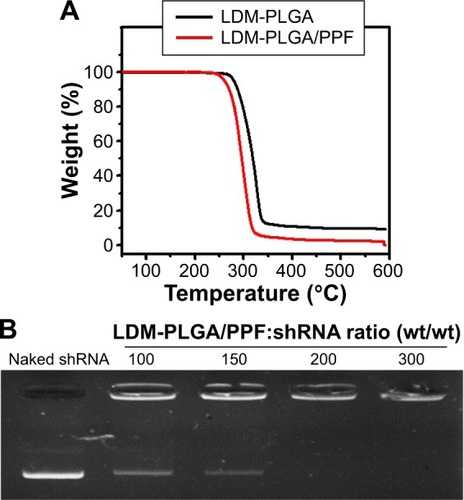
Figure 4 UV-vis and photoluminescent characterization.
Notes: (A) UV-vis absorption (a) and photoluminescence spectra (b) of free DOX and the as-prepared nanoparticles. (B) Photoluminescence spectra of CdSe/ZnS QDs (c) and LM-PLGA (d) (excitation wavelength 490 nm). (C) Photographs of LDM-PLGA/PPF and LD-PLGA/PPF with or without an applied magnetic field. (D) Elemental analysis of LDM-PLGA nanoparticles.
Abbreviations: DOX, doxorubicin; PLGA, poly(d,l-lactic-co-glycolic acid); PPF, PEI-PEG-FA; PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid; QDs, quantum dots.
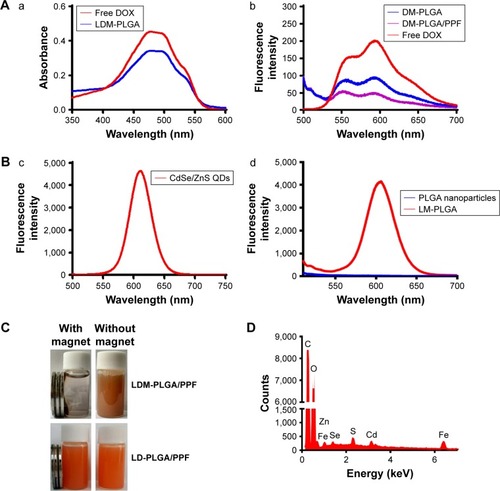
Figure 5 In vitro hemolysis assay. Hemolytic activity of the LM-PLGA (A) and LM-PLGA/PPF (B) at various nanocomposite concentrations (50, 100, 200, 300, and 400 μg/mL, respectively), incubated with rat red blood cells at 37°C for 2 h. Saline and water were used as negative and positive controls, respectively. Insets on the top right are the enlarged UV-vis spectra and bottom-right insets show the photograph of red blood cells treated with LM-PLGA and LM-PLGA/PPF at various concentrations. (C) Optical microscopy images of the dispersion of erythrocytes treated with different nanoparticles. Saline was used as a control.
Abbreviations: PLGA, poly(d,l-lactic-co-glycolic acid); PPF, PEI-PEG-FA; PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid.
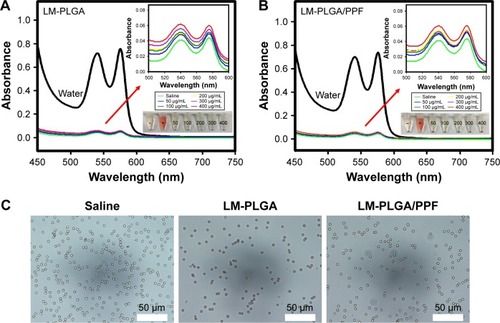
Figure 6 In vitro cell viability of HeLa cells (A) and EMT-6 cells (B) treated with free DOX, LDM-PLGA/PPF, and LM-PLGA/PPF with different DOX concentrations for 48 h with or without preincubation with free folic acid (1.25 mM). (C) Synergistic therapy efficacy of LDM-PLGA/PPF/VEGF shRNA nanocomposites against EMT-6 cells at 25 μg/mL of DOX concentration for 72 h. (D) Fluorescence microscopy images of calcein-AM and propidium iodide costaining of HeLa cells after various treatments at a DOX concentration of 20 μg/mL. Green and red colors represent live and dead cells, respectively. *P<0.05.
Abbreviations: DOX, doxorubicin; PLGA, poly(d,l-lactic-co-glycolic acid); PPF, PEI-PEG-FA; PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid; shRNA, small hairpin RNA; VEGF, vascular endothelial growth factor.
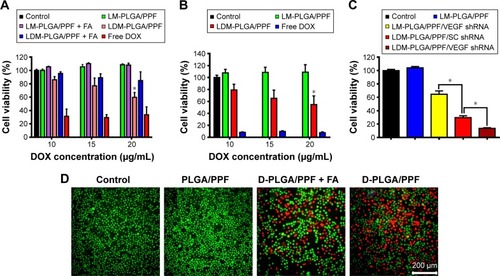
Figure 7 The fluorescence disparity between the samples demonstrated the dynamic interplay of magnetic targeting versus biological targeting via folate under different magnetic fields.
Notes: (A) Fluorescence microscopy images of LDM-PLGA/PPF nanoparticles treated with HeLa cells (a) without and (b) with preincubation with free FA (1.25 mM) before exposure to an external magnetic field for 8 h. (B) Fluorescence microscopy images of HeLa cells with or without preincubation with free FA (1.25 mM) before treatment with LDM-PLGA/PPF nanoparticles for 4 h. (C) The intracellular DOX was quantitatively detected by fluorescence spectrophotometry. *P<0.05 compared with the FA-treated group.
Abbreviations: DOX, doxorubicin; FA, folic acid; PLGA, poly(d,l-lactic-co-glycolic acid); PPF, PEI-PEG-FA; PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid.
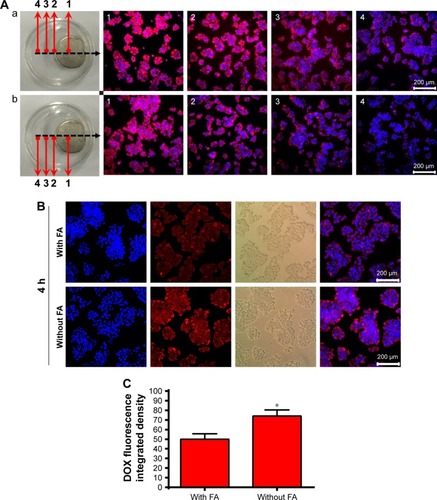
Figure 8 Cellular uptake and endosomal/lysosomal escape of nanocomposites by confocal laser scanning microscopy.
Notes: (A) HeLa cells were treated with free DOX, DM-PLGA, and DM-PLGA/PPF for 6 h. (B) Endosomal/lysosomal escape of DM-PLGA/PPF nanoparticles incubated with HeLa cells at a DOX concentration of 5 μg/mL and 37°C for various time intervals.
Abbreviations: DOX, doxorubicin; DAPI, 4,6-diamino-2-phenylindole; PLGA, poly(d,l-lactic-co-glycolic acid); PPF, PEI-PEG-FA; PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid.
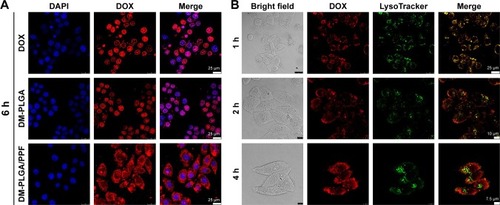
Figure 9 T2-weighted MR images and quantitative signal intensity analysis of LDM-PLGA/PPF.
Notes: (A) T2-weighted MR images and color T2-weighted MR images of HeLa cells treated with LDM-PLGA/PPF at nanoparticle concentrations of 80, 100, 150, 200, and 300 μg/mL (or Fe concentrations of 1.8, 2.3, 3.4, 4.6, and 6.8 μg/mL) for 12 h. The color bar change from red to blue indicates the gradual decrease of MR signal intensity. (B) Quantitative signal intensity analysis. (C) In vivo T2-weighted MR images of EMT-6 tumor-bearing mice before (left) and after (right) injection of LDM-PLGA/PPF. Tumors are marked by the red circle.
Abbreviations: MR, magnetic resonance; PLGA, poly(d,l-lactic-co-glycolic acid); PPF, PEI-PEG-FA; PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid.
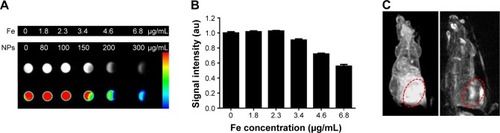
Figure 10 In vivo fluorescence imaging of EMT-6 tumor-bearing mice after intra-tumoral injection of saline (control) or L-PLGA/PPF nanoparticles at 0.5 and 2.5 h.
Notes: The fluorescence signal excitation from CdSe/ZnS quantum dot incorporation into PLGA-based nanoparticles in tumor sites was strong, whereas no apparent fluorescent signal was observed in control mice. The color bar change from red to yellow indicates the gradual increase of fluorescence signal intensity. Tumors are marked by the red circle.
Abbreviations: PPF, PEI-PEG-FA; PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid; PLGA, poly(d,l-lactic-co-glycolic acid).
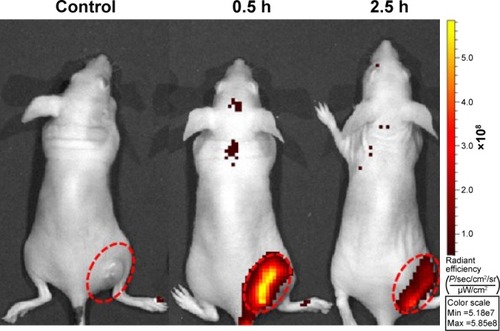
Figure 11 VEGF gene expression.
Notes: (A) The expression of VEGF at the mRNA level in culture media determined by quantitative real-time PCR. (B) The expression of VEGF at the protein level in culture media detected by human VEGF ELISA kit. Both experiments were conducted for a further 72 h after transfection with Lipo/VEGF shRNA, PLGA/PPF/VEGF shRNA, or PLGA/PPF/SC shRNA. The weight ratio of nanocomposites to SC shRNA or VEGF shRNA was 150:1. *P<0.05 compared with control.
Abbreviations: PLGA, poly(d,l-lactic-co-glycolic acid); PPF, PEI-PEG-FA; PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid; SC shRNA, scrambled small hairpin RNA; VEGF, vascular endothelial growth factor.
Figure 12 In vivo antitumor activity.
Notes: (A) Tumor growth curves of the various treatment groups, with the treatment schedule indicated by the red arrows. (B) Body weight changes in mice following the various treatments. (C) Optical images of tumors of different treatment groups: (a) saline, (b) LM-PLGA/PPF, (c) LM-PLGA/PPF/VEGF shRNA, (d) LDM-PLGA/PPF/SC shRNA, and (e) LDM-PLGA/PPF/VEGF shRNA. (D) Weight of dissected tumor tissues from the mice of five groups after the last treatment and sacrifice. (E) Representative H&E-stained tumor sections from the mice of the five treatment groups: (a) saline, (b) LM-PLGA/PPF, (c) LM-PLGA/PPF/VEGF shRNA, (d) LDM-PLGA/PPF/SC shRNA, and (e) LDM-PLGA/PPF/VEGF shRNA on BALB/c mice bearing EMT-6 tumors after the last treatment and sacrifice. *P<0.05 and **P<0.01 compared with the control (saline treatment).
Abbreviations: PLGA, poly(d,l-lactic-co-glycolic acid); PPF, PEI-PEG-FA; PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid; shRNA, small hairpin RNA; VEGF, vascular endothelial growth factor.
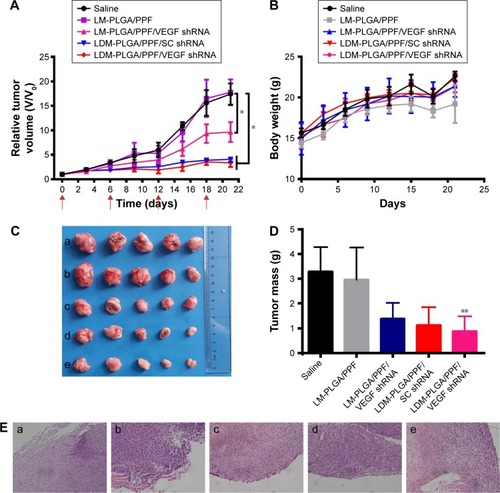
Figure S1 1H NMR spectra of PEG-FA (A) and PEI-PEG-FA (B).
Abbreviation: PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid.
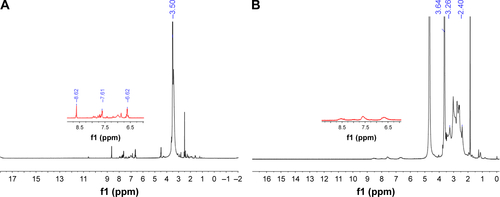
Figure S2 In vitro release of DOX from LDM-PLGA (A) and LDM-PLGA/PPF (B) under different pH conditions and time points. Drug concentration in the supernatant was assessed by measuring the absorbance at 480 nm.
Abbreviations: DOX, doxorubicin; PLGA, poly(d,l-lactic-co-glycolic acid); PPF, PEI-PEG-FA; PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid.
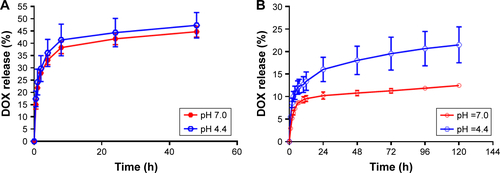
Figure S3 Stability tests.
Notes: (A) Images of LDM-PLGA/PPF dispersed in PBS, saline, serum-free RPMI 1640 cell culture medium, and complete RPMI 1640 cell culture medium. (B) Hydrodynamic size and zeta potential of LDM-PLGA/PPF at different time points.
Abbreviations: PLGA, poly(d,l-lactic-co-glycolic acid); PPF, PEI-PEG-FA; PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid; PBS, phosphate-buffered solution; RPMI, Roswell Park Memorial Institute.
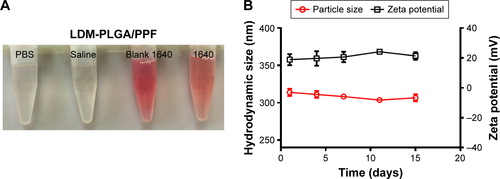
Figure S4 In vitro cell viability of HeLa cells and HUVEC after treatment LM-PLGA/PPF at the concentration of 0–250 μg/mL for 48 (A) and 72 h (B).
Abbreviations: HUVEC, human umbilical vein endothelial cell; PLGA, poly(d,l-lactic-co-glycolic acid); PPF, PEI-PEG-FA; PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid.
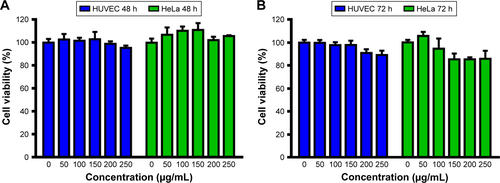
Figure S5 In vitro representative fluorescence images by activation of CdSe/ZnS quantum dots.
Abbreviations: PLGA, poly(d,l-lactic-co-glycolic acid); PPF, PEI-PEG-FA; PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid; PBS, phosphate-buffered solution.
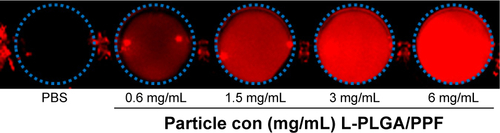
Figure S6 Photographs of different groups of tumor-bearing mice after 21-day treatment with saline, LM-PLGA/PPF, LM-PLGA/PPF/VEGF shRNA, LDM-PLGA/PPF/SC shRNA, and LDM-PLGA/PPF/VEGF shRNA.
Abbreviations: PLGA, poly(d,l-lactic-co-glycolic acid); PPF, PEI-PEG-FA; PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid; shRNA, small hairpin RNA; VEGF, vascular endothelial growth factor.
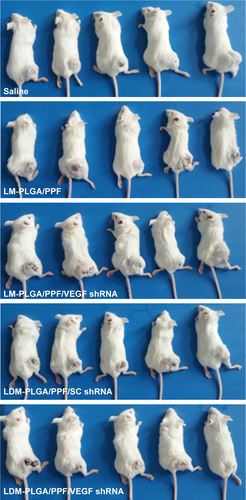
Figure S7 Representative images of H&E-stained sections of the heart, liver, spleen, lung, and kidney collected from tumor-bearing BALB/c mice after treatment with saline (A), LM-PLGA/PPF (B), LM-PLGA/PPF/VEGF shRNA (C), LDM-PLGA/PPF/SC shRNA (D), and LDM-PLGA/PPF/VEGF shRNA (E).
Abbreviations: PLGA, poly(d,l-lactic-co-glycolic acid); PPF, PEI-PEG-FA; PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid; SC shRNA, scrambled small hairpin RNA; VEGF, vascular endothelial growth factor.
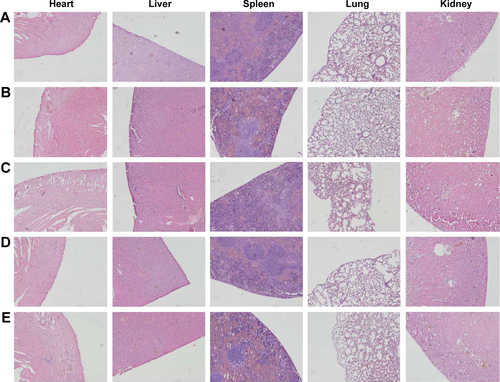
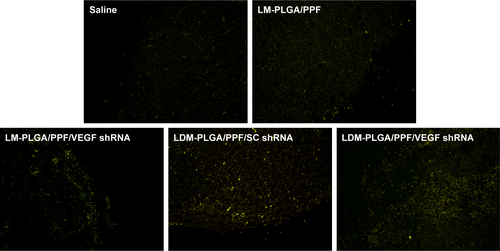
Figure S8 TUNEL staining of the tumors with different treatments.
Note: TUNEL-positive cells (apoptotic cells) exhibited yellow-green fluorescence.
Abbreviations: PLGA, poly(d,l-lactic-co-glycolic acid); PPF, PEI-PEG-FA; PEI-PEG-FA, polyethyleneimine premodified with polyethylene glycol-folic acid; SC shRNA, scrambled small hairpin RNA; TUNEL, terminal deoxynucleotidyl transferase dUTP nick-end labeling; VEGF, vascular endothelial growth factor.