Figures & data
Figure 1 α-Tocopherol derivative with an isoprenoid side chain shortened to 6 carbon atoms in (A) phenolic and (B) acetate forms.
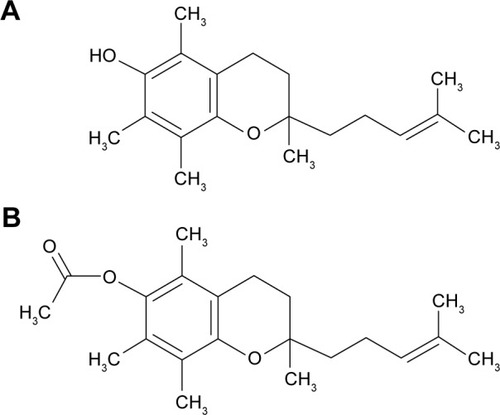
Figure 2 Transmission electron micrographs of (A) γ-Fe2O3 and (B) γ-Fe2O3@PDMA nanoparticles before and (C) after incubation in 0.1 M HCl (pH 3) at 37°C for 1 h, and (D) CuFe2O4 nanoparticles.
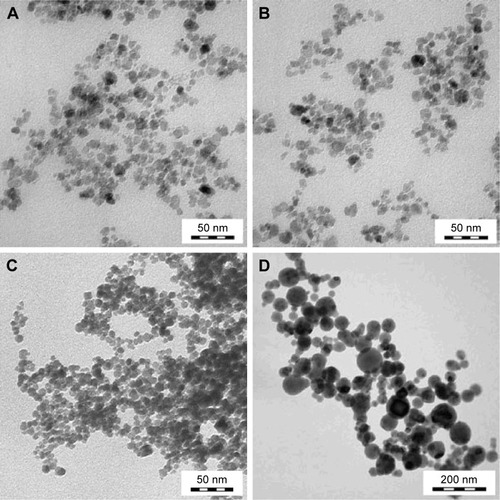
Figure 3 (A) FTIR spectra of (1) PDMA, (2) γ-Fe2O3@PDMA, and (3) γ-Fe2O3 nanoparticles. (B) TGA of (1) γ-Fe2O3 and (2) γ-Fe2O3@PDMA nanoparticles. (C) SQUID measurements of (1) γ-Fe2O3, (2) γ-Fe2O3@PDMA, and (3) CuFe2O4 nanoparticles.
Abbreviations: TGA, thermogravimetric analysis; SQUID, superconducting quantum interference device; PDMA, poly(N,N-dimethylacrylamide); FTIR, Fourier transform infrared.
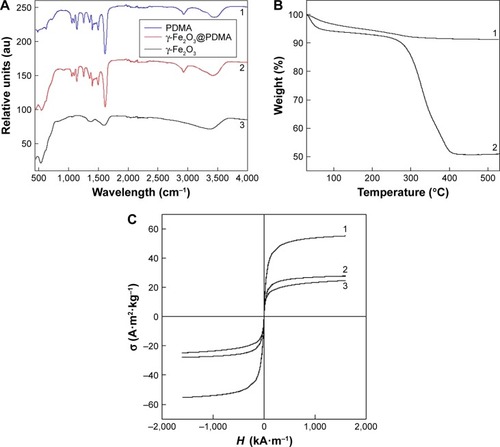
Figure 4 In vitro Fe2+ release from (A) CuFe2O4, (B) γ-Fe2O3, and (C) γ-Fe2O3@PDMA nanoparticles in the presence of different concentrations of (♦) Toc and (■) Toc-6-OH. Nanoparticles (250 µg/mL) were incubated in a 0.9% NaCl solution and 10 mM Tris (pH 7.4) at 37°C for 24 h. Data are presented as the mean ± SE (n=5–8). *Significantly different from the absence of Toc and Toc-6-OH. #Significantly different from Toc.
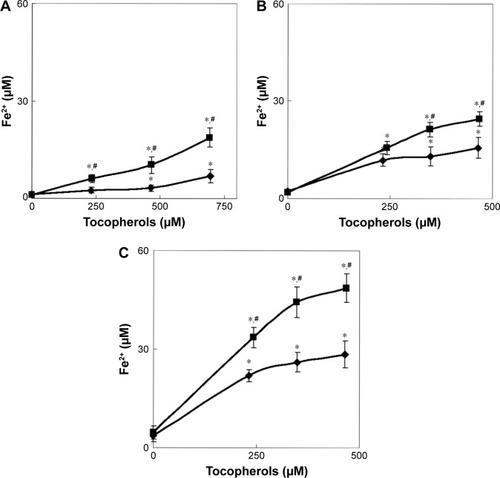
Figure 5 In vitro lipid oxidation in the blood serum in the (1) absence or (2–4) presence of CuFe2O4, (5–7) γ-Fe2O3, and (8–10) γ-Fe2O3@PDMA nanoparticles incubated at 37°C for 24 h; (2, 5, 8) 4.4, (3, 6, 9) 44, and (4, 7, 10) 444 µg of particles per milliliter. Data are presented as the mean ± SE (n=5–8). *Significantly different from (1).
Abbreviations: TBARS, thiobarbituric acid reactive species; PDMA, poly(N,N-dimethylacrylamide).
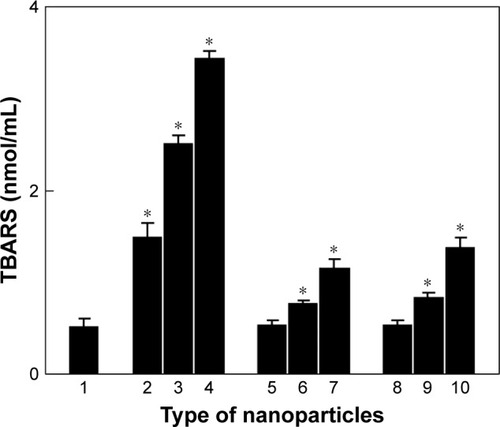
Figure 6 Antitumor effect of γ-Fe2O3@PDMA nanoparticles on Walker-256 mammary gland carcinosarcoma in Wistar rats. From left to right: untreated rat with a tumor (control), rat with a tumor treated with intraperitoneally administered doxorubicin, and rat with a tumor treated with γ-Fe2O3@PDMA nanoparticles administered per os.
Abbreviation: PDMA, poly(N,N-dimethylacrylamide).
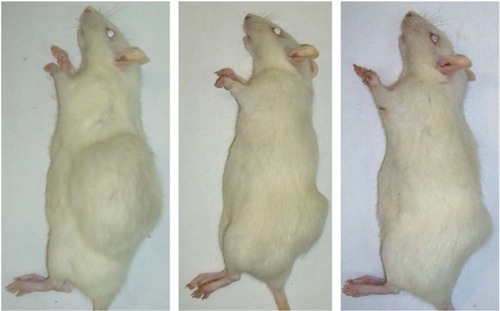
Figure 7 In vivo antitumor activity of nanoparticles on Walker-256 mammary gland carcinosarcoma in Wistar rats. (1) No treatment (control), treatment with (2) 1.5 mg of doxorubicin, (3) 25 mg of Toc-6-Ac, (4) 10 mg of CuFe2O4, (5) 10 mg of γ-Fe2O3, (6) 10 mg of γ-Fe2O3@PDMA, (7) 10 mg of CuFe2O4 + 25 mg of Toc-6-Ac, (8) 10 mg of γ-Fe2O3 + 25 mg of Toc-6-Ac, or (9) 10 mg of γ-Fe2O3@PDMA + 25 mg of Toc-6-Ac per kilogram of body weight. Data are presented as the mean ± SE (n=5–7). *Significantly different from (1) control animals; #significantly different from 2) animals treated with doxorubicin.
Abbreviation: PDMA, poly(N,N-dimethylacrylamide).
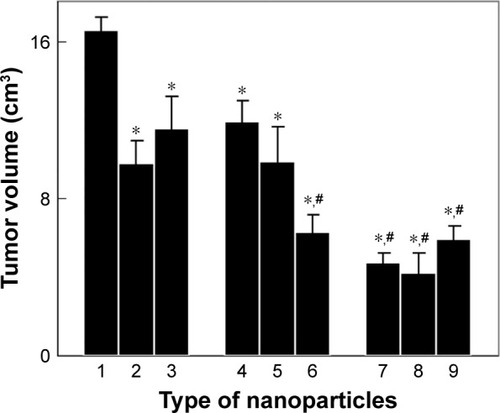
Figure 8 In vivo determination of hepatic function using (A) ALT, (B) AST, and (C) bilirubin analyses of the blood plasma of Wistar rats with Walker-256 mammary gland carcinosarcoma. (1) No treatment (tumor control), treatment with (2) 1.5 mg of doxorubicin, (3) 25 mg of Toc-6-Ac, (4) 10 mg of CuFe2O4, (5) 10 mg of γ-Fe2O3, (6) 10 mg of γ-Fe2O3@PDMA, (7) 10 mg of CuFe2O4 + 25 mg of Toc-6-Ac, (8) 10 mg of γ-Fe2O3 + 25 mg of Toc-6-Ac, or (9) 10 mg of γ-Fe2O3@PDMA + 25 mg of Toc-6-Ac kilogram of body weight, and (10) non-treated rats without a tumor (intact control). Data are presented as the mean ± SE (n=5–7). *Significantly different from (1) control animals; #significantly different from (4–6) animals treated with neat nanoparticles.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; PDMA, poly(N,N-dimethylacrylamide).
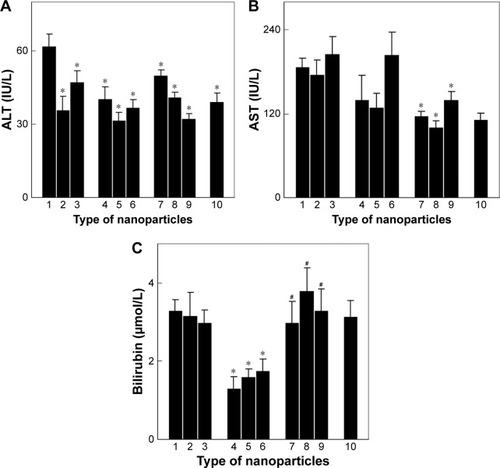
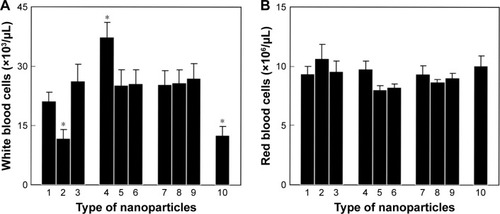
Figure 9 In vivo complete blood count of (A) white and (B) red blood cells in Wistar rats with Walker-256 mammary gland carcinosarcoma. (1) No treatment (control), treatment with (2) 1.5 mg of doxorubicin, (3) 25 mg of Toc-6-Ac, (4) 10 mg of CuFe2O4, (5) 10 mg of γ-Fe2O3, (6) 10 mg of γ-Fe2O3@PDMA, (7) 10 mg of CuFe2O4 + 25 mg of Toc-6-Ac, (8) 10 mg of γ-Fe2O3 + 25 mg of Toc-6-Ac, or (9) 10 mg of γ-Fe2O3@PDMA + 25 mg of Toc-6-Ac per kilogram of body weight, and (10) non-treated rats without a tumor (intact control). Data are presented as the mean ± SE (n=5–7). *Significantly different from (1) control animals.
Abbreviation: PDMA, poly(N,N-dimethylacrylamide).