Figures & data
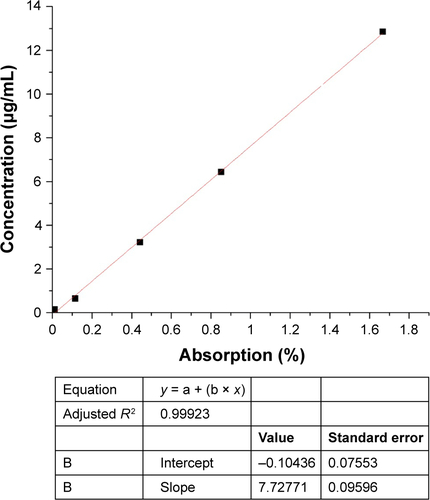
Figure 1 Preparation process of CM-SF NPs via SEDS.
Notes: SF powder and CM powder were mixed and dissolved in HFIP solution, ready for the SEDS process. After complete removal of HFIP solution, CM-SF NPs were collected. Yellow curves and circles, curcumin; blue curves, silk fibroin; blue oval, HFIP.
Abbreviations: CM, curcumin; SF, silk fibroin; NPs, nanoparticles; SEDS, solution-enhanced dispersion by supercritical CO2; HFIP, hexafluoroisopropanol.
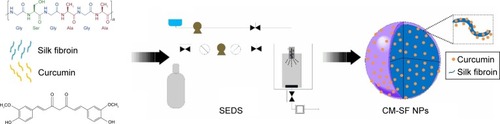
Figure 2 Physicochemical characterization of CM-SF NPs.
Notes: (A) SEM images of original CM powders (inset, particle size and distribution of original CM powders); (B) SEM images of CM-SF NPs prepared by SEDS CO2 (inset, particle size and distribution of CM-SF NPs); (C) FTIR spectra of SF, CM-SF NPs, and original CM powders; (D) solubility comparison of CM-SF NPs and original CM powders; (E, F) optical images of CM-SF NP solution and original CM-powder solution after 1 hour dissolution.
Abbreviations: CM, curcumin; SF, silk fibroin; NPs, nanoparticles; SEM, scanning electron microscopy; SEDS, solution-enhanced dispersion by supercritical CO2; FTIR, Fourier-transform infrared spectroscopy.
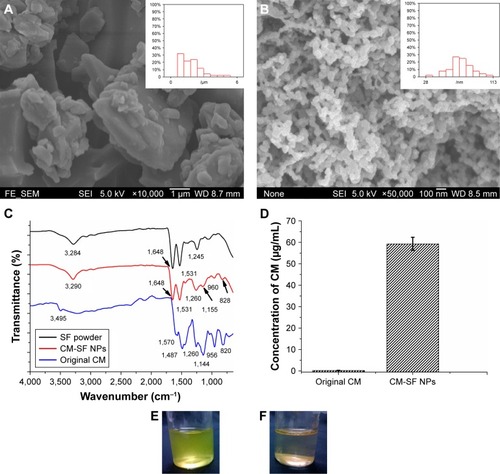
Figure 3 Fluorescence images and endocytosis pathway.
Notes: (A) Images show intracellular uptake efficiency of colon cancer cells (HCT116) after 0.5 and 6 hours’ treatment with CM-SF NPs, CM-DMSO (original CM powders dissolved in DMSO solution), and control groups; (B) endocytosis pathway mechanism that enhanced cellular uptake efficiency of CM-SF NPs.
Abbreviations: CM, curcumin; SF, silk fibroin; NPs, nanoparticles; DMSO, dimethyl sulfoxide; DAPI, 4′,6-diamidino-2-phenylindole.
Figure 4 Smart-treatment potential of CM-SF NPs on colon cancer cells.
Notes: (A) In vitro anticancer effect (dose-dependent) of CM-SF NPs on colon cancer HCT116 cells; (B) cytotoxicity of CM-SF NPs on normal NCM460 cells; (C) smart-treatment concentration range of CM-SF NPs, in which the growth of cancer cells was strongly inhibited, while the growth of normal cells was minimally inhibited. Results are shown as means ± SD, n=6. ***P<0.001.
Abbreviations: CM, curcumin; SF, silk fibroin; NPs, nanoparticles; DMSO, dimethyl sulfoxide; 5-Fu, fluorouracil.
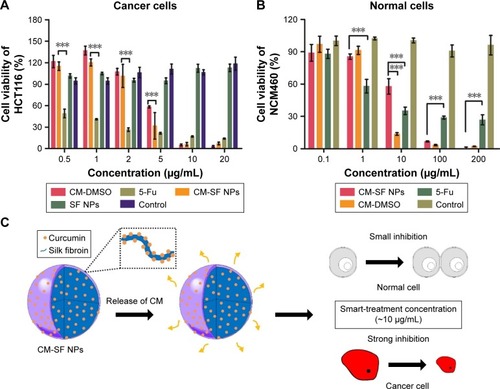
Figure 5 Anticancer mechanism of CM-SF NPs against colon cancer cells.
Notes: (A) Qualitative cell-cycle and (B) apoptotic progression of HCT116 cells in response to control, 5-Fu, CM-SF NPs, and CM-DMSO treatment for 24 hours. Quadrants Q1, Q2, Q3, and Q4 reflect necrosis, late apoptosis, and early apoptosis and live, respectively. (C) Quantitative analysis of cell-cycle and (D) FACS distributions (%) of apoptotic HCT116 cells in response to different groups at 24 hours. Total apoptosis includes late apoptosis plus early apoptosis. All data were obtained from at least three independent experiments. *P<0.05; **P<0.01; ***P<0.001.
Abbreviations: CM, curcumin; SF, silk fibroin; NPs, nanoparticles; DMSO, dimethyl sulfoxide; FACS, fluorescence-activated cell sorting; 5-Fu, fluorouracil.
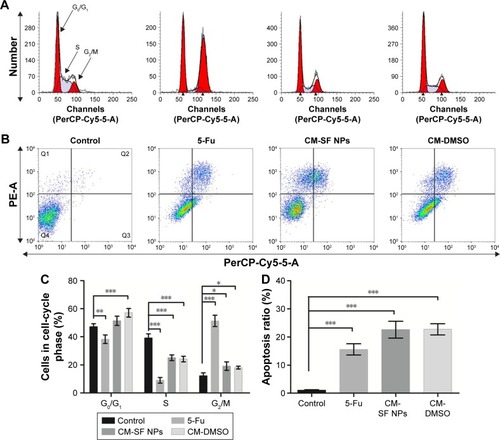
Figure S1 Solution-enhanced dispersion by sc-CO2 equipment.
Notes: 1, Solution pump; 2, precipitation vessel; 3, gas bath; 4, CO2 cylinder; 5, cooler; 6, CO2 pump; 7, heat exchanger; 8, specially designed coaxial nozzle; 9, gas filter; 10, water bath.
Abbreviations: P, pressure gauge; sc, supercritical; T, thermometer.
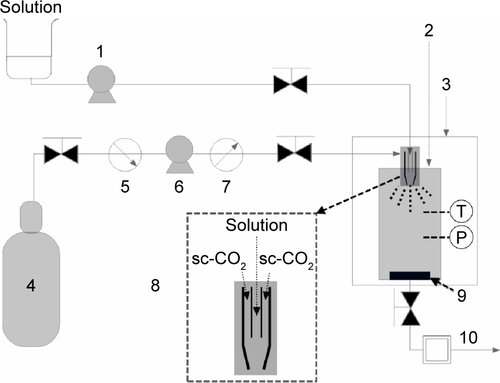
Figure S3 Cell proliferation of HCT116 after treatment with CM-SF NPs, CM-DMSO, 5-Fu, SF NPs, and control.
Note: Results are shown as means ± SD, n=6.
Abbreviations: CM, curcumin; SF, silk fibroin; NPs, nanoparticles; DMSO, dimethyl sulfoxide; 5-Fu, 5-flurouracil.
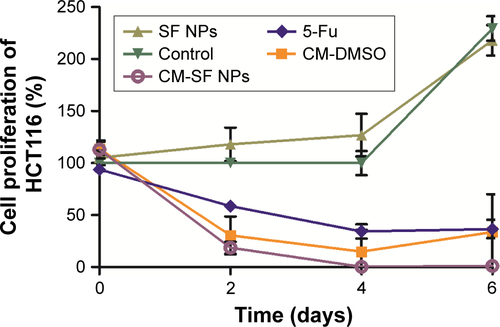
Table S1 IC50 comparison of samples